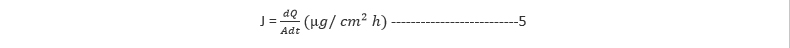1. Introduction
Transdermal drug delivery systems are basically self-contained, discrete dosage forms [1] which when applied to an intact skin delivers therapeutically active substances through the skin at a controlled rate to the systemic circulation [2]. These devices are medicament-containing patches applied topically for systemic effect at a predetermined rate [3]. Some of the major advantages of transdermal drug delivery are that it provides controlled release of the drug to the patient, enables a steady blood level profile, prevents first-pass hepatic metabolism and can provide rapid termination of therapy [4]. Transdermal administration of drugs has the benefits of impeding drug metabolism or chemical degradation in the gastrointestinal tract [5,6].
Polymers can be used as matrices for transdermal devices. The polymer structure, size and shape determine the properties of the polymer. These polymers may be hydrogels which are usually cross-linked macromolecular networks of polymers. The process of drug release in most controlled-release devices including transdermal films or patches is governed by diffusion and the polymer matrix affects the drug diffusivity as the movement of the drug molecules is restricted by the three-dimensional network of polymer chains. Proportionally different mixtures of polymers cause alterations of the cross-linking and modification of structural arrangements of the polymers [7] which may be explored to create varied physical, chemical, mechanical and permeability properties for a range of products. Hydroxypropyl methylcellulose, polyvinyl alcohol and cassava starch are polymeric materials with diverse qualities and can be applied in transdermal technology. Diazepam is a benzodiazepine drug that is commonly used as sedative, muscle relaxant and anticonvulsant. Diazepam can be incorporated in transdermal matrix systems to enable rapid termination of therapy when need arises or for preventive therapy.
The aim of this study is to investigate the physico-chemical, mechanical and permeation characteristics of diazepam transdermal delivery patches.
2 Materials and methodology
2.1 Materials
Diazepam (A gift from Pauco Pharmaceuticals), hydroxypropyl methylcellulose (Qualifine Chemicals, India), PEG 6000, polyvinyl alcohol (Merck, Germany), propylene glycol, Tween® 80 (Sigma Aldrich, USA), cassava starch (extracted in Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria Nsukka).
2.2 Extraction of cassava starch
The tubers of Manihot utilissima (cassava) were peeled and reduced into small pieces. The reduced pieces of cassava tubers were soaked in 0.1 M sodium metabisulphite solution for 48 h. Thereafter, the metabisulphite solution was washed off and pieces of cassava tubers ground/ blended to a pulp. The cell debris was removed by passing the slurry through a muslin cloth of 150 mm aperture sieve size, with occasional passage of water for effective filtration. The starch was thereafter allowed to settle and the supernatant decanted. The starch was suspended in 0.1 N potassium hydroxide solution for 12 h. The solution was washed off completely and the starch suspended in 0.1 N sulphuric acid solution for 12 h. The solution was effectively washed off and the settled starch washed with distilled water, and dried at 40 °C for 6 h in an oven. The starch was free from significant amounts of cyanide.
2.3 Characterization cassava starch
Starch was identified using microscopic evaluation, mucilage formation, iodine and organoleptic observations.
2.3.1 Microscopic evaluation of particle size and morphology
The particle size and morphology were studied by viewing thin films of dispersions under a light microscope and images captured with a Motic® camera (moticam) and analysed using Motic® images plus 2.0 software.
2.3.2 Organoleptic Evaluation, iodine staining and mucilage formation test
A 2g quantity of the cassava starch powder was examined virtually for colour examination. A pinch of the weighed powder was rubbed between the index finger and the thumb to access the texture, and the sample was also observed for odour.
A weighed 1 g quantity of the extracted tapioca starch was transferred into a test tube. Three drops of iodine solution were introduced into the test-tube and the sample observed for the appearance of a blue-black colouration.
Furthermore, a 5 g quantity of the extracted starch sample was weighed and transferred into a 50 ml beaker. A 2 ml volume of water, pre-heated to 60 oC, was measured and poured into the beaker followed by thorough stirring with a glass stirrer until uniform and consistent mix was obtained which was observed.
2.4 Preparation of diazepam transdermal patches
The diazepam transdermal patches were formulated by the solvent casting technique. Varying concentrations of hydroxypropyl methylcellulose, cassava starch, polyethylene glycol 8000 and Tween® 80 were employed. Three different templates were applied.
2.4.1 Preparation of transdermal patches based on HPMC and cassava starch as binary film-formers
For Batch HC4, HPMC and cassava starch blend (4:1) was prepared by weighing out 4g of HPMC and 1g of cassava starch and mixing them thoroughly. The blend was transferred into a 250 ml beaker and was dispersed in 10 ml of water heated to 60 oC. This was followed by thorough mixing with a stirrer until mucilage of uniform consistency was obtained. Thereafter, 20ml of water was used to dilute the mucilage followed by further mixing.
Separately, 500 mg of diazepam, 1g of PEG 6000, 0.05 ml of Tween® 80 and 1ml of propylene glycol were dispersed in 10 ml of water at room temperature. This dispersion was then added to the HPMC/cassava starch mucilage, followed by continuous stirring. The mucilage was diluted with water to 50 ml and the resulting dispersion was further stirred for 5 min and thereafter poured into petri dish of defined area (8 cm2). The solvent was allowed to evaporate over 48 h at room temperature to obtain dry patches (HC4). The products were stored between sheets of wax paper in a desiccator containing anhydrous calcium chloride (CaCl2) as the desiccant until further use.
Another batch was prepared with HPMC and cassava starch blend (3:2) by weighing out 3g of HPMC and 2g of Cassava starch and mixing them thoroughly using a mixer. The remaining steps as above were repeated to obtain dry patches (HC3).
2.4.2 Preparation of transdermal patches based on HPMC and cassava starch using methanol as solvent
A 80 ml quantity of methanol/water (9:1) solution was prepared by measuring 8 ml of water and pouring into a conical flask containing 72 ml of methanol followed by proper corking to prevent solvent evaporation. 5g of the film forming agent, HPMC was weighed and dispersed in 40 ml of the methanol/water (9:1) solution followed by proper stirring. Thereafter, 500 mg of diazepam, 2 g of PEG were weighed and added to the HPMC dispersion followed by thorough stirring. Subsequently, 0.05 ml of Tween 80, and 5 ml of propylene glycol were added to the mixture and this was followed by further stirring. The dispersion above was made up to 50 ml in volume and was further stirred for 5 min and thereafter poured into petri dish. A funnel of suitable size was inverted over the petri dish to control solvent evaporation. The casting solvent was then allowed to evaporate over 48 h at room temperature to obtain dry patches (HM). The products were stored in a desiccator containing anhydrous calcium chloride (CaCl2) as the desiccant until further use.
Other batches were prepared using mixtures of HPMC and cassava at 4:1 (HCM4) and 3:1 (HCM3) ratios.
2.4.3 Preparation of transdermal patches based on polyvinyl alcohol
An 8 g quantity of PVA was weighed and dispersed in 40 ml of water inside a 250 ml beaker. The dispersion was heated over a water bath at 90oC for 5 min. A gel consistency was formed on mixing with water and this gel became less viscous on heating. The mixture was allowed to cool to 40oC and then a 500 mg quantity of diazepam was weighed and added, followed by thorough stirring for 5 min. The formulation was transferred into clean petri dish. The solvent was then allowed to evaporate over 24 h at room temperature to obtain dry patches (batch P8). The products were stored in a desiccator containing anhydrous calcium chloride (Cacl2) as the desiccant until further use. This procedure was repeated, but using 6g and 4g quantities of PVA to form batches P6 and P4, respectively.
2.5 Characterization of diazepam transdermal patches
2.5.1 Fourier transform infrared spectroscopy (FT-IR)
FT-IR was used to investigate possible chemical interaction between diazepam and transdermal patch excipients. Sufficient quantities of pure diazepam, HPMC, cassava starch, polyethylene glycol 6000, polyvinyl alcohol, and the formulated transdermal patches were scanned over a wavelength range of 4000 to 400 cm-1 at a resolution of 16 in a FT-IR instrument (Shimadzu cooperation model IR Affinity-1, Japan). The system was operated in the absorbance mode. Samples were placed on potassium bromide (KBr) sample stage in which solid samples were mixed with KBr.
2.5.2 Physical appearance and properties of transdermal patches
All the formulated patches were inspected for clarity, colour, odour and smoothness. These physical attributes involved use of sense organs.
2.5.3 Thickness uniformity and folding endurance of patches
The thickness of each patch was measured at different points of the films using a Vernier calliper and the average and variations calculated accordingly.
Folding endurance was determined manually by folding a 1 cm2 strip of each patch repeatedly at the same point on the patch until a breaking point was reached. The number of times that the patch could be folded at the same point without breaking or cracking, was taken as the value for the folding endurance.
2.5.4 Percentage moisture uptake and loss of patches
The percentage moisture uptake of the patches was determined using static methods involving saturated salt solutions. For moisture uptake, weighed patches were placed in desiccators containing saturated solution of potassium chloride (84% relative humidity RH). At 24 h intervals, the patches were removed and weighed until there was no observable change in weight.
The percentage moisture uptake was then calculated as one hundred times the ratios of the difference between final and initial weights to the initial weights of the patches. This is mathematically presented in Equation 1
 For percentage moisture loss, the weighed patch samples were kept in a desiccator containing anhydrous calcium chloride. At 24 h interval, the patches were removed and weighed repeatedly until there was no observable change in weight. The percentage moisture loss was then calculated from the difference between initial and final weight with respect to initial weight as presented in Equation 2:
For percentage moisture loss, the weighed patch samples were kept in a desiccator containing anhydrous calcium chloride. At 24 h interval, the patches were removed and weighed repeatedly until there was no observable change in weight. The percentage moisture loss was then calculated from the difference between initial and final weight with respect to initial weight as presented in Equation 2:
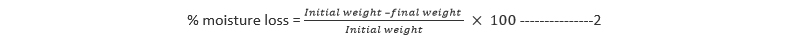
2.5.5 Percentage elongation break test.
The percentage elongation break was determined by recording the length just before the break point after elongation or stretching. A 2 × 0.5 cm sample was cut from each batch and the elongation test performed on them.
The percentage elongation was determined from Equation 3. Highly elastic materials tend to have higher values.
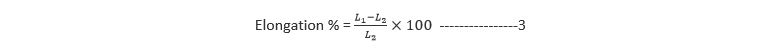
Where L1 and L2 are the final and initial lengths of each strip
2.5.6 Swelling Index (S.I)
A 1 X 1 cm2 strip of the transdermal patches was cut and dried until constant weight (W1) using desiccator containing anhydrous calcium chloride at room temperature for one day. The patches were immersed in 100 ml of distilled water at 37 °C and reweighed after removal of excess water by pressing it gently between two filter papers (W2). The re-weighed patches were retained in the desiccators and allowed to be dried to constant weight (W3). Swelling index (S.I) was calculated from Equation 4
Where W2 is the weight of immersed patch and W3 is the weight of re-dried patch.
2.5.7 Ex vivo skin permeation studies
A modified, novel, ex vivo model was applied in the study (Agubata and Ottah Technique). The formulated transdermal patches (1×1 cm2) were separately stuck and ‘sandwiched’ between the excised rat skin and effectively sealed. The skin- enclosed patches were then suspended in 0.1 N HCl medium (volume, 100ml) which served as the receptor compartment. The whole assembly was placed on a magnetic stirrer apparatus and the solution in the receptor compartment continuously stirred using magnetic beads at 50 rpm, while the temperature was maintained at 37+ 1°C. Ten millilitre samples were withdrawn at 10 min intervals for 2 hours and analysed for drug content using a UV/VIS spectrophotometer (Jenway 6405, England) at 284 nm. The receptor phase was replenished with equal volume of 0.1N HCl solution (10 ml), after each sample withdrawal to maintain a sink condition. The cumulative percentage of drug permeated per square centimetre of patches was plotted against time.
The permeation data was analysed by determining the flux of the drug. The flux (µg cm-2 h-1) of diazepam was calculated from the slope of the plot of the cumulative amount of diazepam permeated per cm2 of skin at steady state against time using linear regression analysis [8,9]. The steady-state flux (J) was determined from the relationship in Equation 5:
Where Q indicates the quantities of substances crossing the rat skin, A is the area of the rat skin exposed and t is the time of exposure.
The permeation coefficients are obtained from the steady-state flux values using Equation 6:
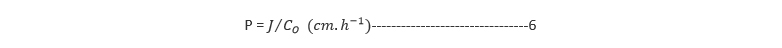
Where P is the permeation coefficient, Co is the initial drug concentration in the donor compartment and J represents the steady-state flux obtained from Equation 5. Co is the concentration of drug in the patch.
3 Results and Discussion
3.1 Characterization of the extracted Cassava starch
3.1.1 Microscopic property and identification of cassava starch:
The microscopic view of the extracted cassava starch sample revealed spherical particles with average diameter of 13.27 ± 1.79µm. Furthermore, the photomicrograph showed that the cassava starch granules were monodispersed with relatively equal particle sizes. This is shown in Fig 1.
The cassava starch was positively identified using routine tests.
3.2 Fourier transform infrared spectroscopy
The FT-IR results showed that there was no drug-excipient interaction between the ingredients used for the transdermal formulations since all the bands observed in the formulations represented functional groups in the individual components.
3.3 Characterization of the Transdermal Patches
Physically, the patches were generally opaque, white, relatively smooth and odorless.

Click to view
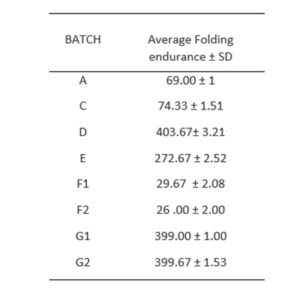
3.3.1 Thickness uniformity and folding endurance
The films exhibited average thickness of 2 mm. The folding endurance test (Table 1) showed that films prepared with polyvinyl alcohol exhibited the highest endurance of around 404 folding cycles whereas the least endurance of around 26 cycles were observed with patches/ films prepared with HPMC/cassava starch using methanol as solvent. The high folding endurance of the PVA-based hydrogels may be attributed to its high physical cross-link and partial self-healing capabilities. These properties are favoured by concentration-dependent interchain H-bonding and diffusion of PVA chains over fractured surfaces [10].
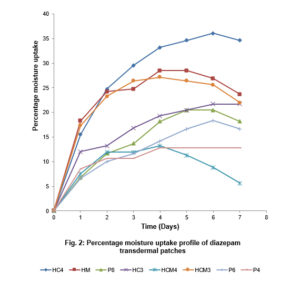
3.3.2 Percentage Moisture Uptake and Loss
The percentage moisture uptake of the formulated patches observed over time (days) are presented in Fig. 2. Patches prepared with HPMC and cassava starch at 4:1 ratio showed highest percentage moisture uptake (36% max. moisture uptake). Its gel network seems to encourage moisture absorption. Patches prepared with HPMC showed higher moisture absorptive capacity than those prepared with PVA. Within 4 to 6 days, most patches showed optimum moisture uptake and experienced equilibrium or drops in their % moisture uptake afterwards. In the figure, this relationship is graphically presented. The differences in moisture uptake profile show that the percent moisture uptake by the formulated transdermal patches are related to the type and amount of excipients used in the formulation.
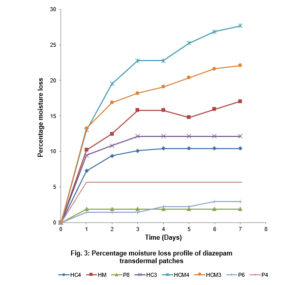
Under the specified conditions in a desiccator containing anhydrous calcium chloride, all patch samples showed significant loss of moisture content within days, with patches HCM4 (prepared with HPMC/Cassava starch 4:1 and methanol) and Polyvinyl alcohol batches showing highest and lowest percentage moisture losses, respectively. This is represented graphically in Fig. 3. The differences in moisture uptake and loss of the materials may be related to properties which include sorption, swelling capacity and hydrogen bonding that are dependent on material. The strongly cross-linked network of polyvinyl alcohol may be responsible for its relatively lower moisture loss.
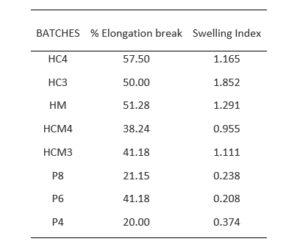
3.3.3 Percentage Elongation Break Test
The percentage elongation break test measures the ability of the formulated patches to withstand mechanical stress of pull without breaking. From the results (Table 2) obtained, it is observed that batch HC4 sample showed highest percentage elongation of 57.5%, thus higher elasticity, while sample batches P4 and P8 showed least percentage elongations of 20 and 21%, respectively. Films prepared with polyvinyl alcohol (especially those formulated with 4 and 8g) showed the least percentage elongation which imply that they have relatively lower elasticity.
3.3.4 Swelling Index
In the swelling index results, the formulated transdermal films based on hydroxypropyl methyl cellulose showed significantly higher (p<0.05) swelling index than the patches prepared with polyvinyl alcohol. This result validates the observations in moisture uptake studies since a similar pattern was observed where absorption of water is favoured by HPMC-based patches. Films prepared with HPMC and cassava starch mixture at 3:1 ratio had swelling index of 1.85 which was the highest value. The lowest value (0.208) was observed in those prepared with 6g of PVA. This is presented in Table 2.
Click to view
Click to view
Click to view
Click to view
3.3.5 Ex-vivo skin permeation studies
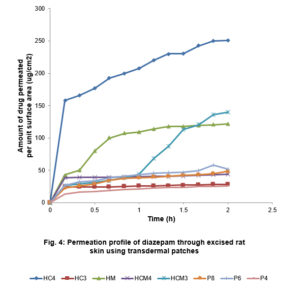
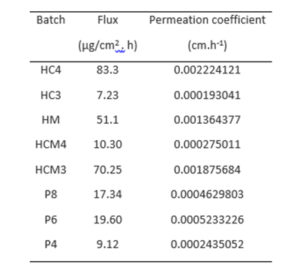
The amount of drug permeated per unit surface area was plotted against time and presented in Figure 4. Transdermal films prepared with HPMC and cassava starch (4:1 mixture) (HC4) showed the highest diazepam permeation, flux (83.3 µg/cm2 h) and permeation constant (0.0022cm.h-1) (p<0.05). Same mixtures prepared with methanol (at 3:1 ratio) also released the drug which permeated the excised rat skin at 70.25 µg /cm2 h. The order of decreasing flux is HC4>HCM3>HM>P6>P8>HCM4>P4>HC3. Although the permeation order did not reveal any definite pattern, HPMC-based films showed much higher drug release and permeation. However, the pattern across the batches shows that a critical ratio for efficiency is required for both aqueous-based and methanol-based preparations.
For all the batches, there was an initial high drug release which was very significant with the HC4.
Earlier reports have shown that rat skin is much more permeable to drugs than human skin with the permeability of rat skin estimated to be about ten times more [11].
The permeability of the drug depends on the molecular weight of the drug, skin thickness and partition coefficient, all of which predicts permeability using an empirical model. On the other hand, the mechanistic approach to prediction of drug permeability uses skin compartments and diffusion pathways [12].
The quantity of drug that diffused through the rat skin is also dependent on the nature of the matrix system holding the drug. The monolith determines the rate of drug it releases which would also determine the amount that eventually permeates. The matrix-based transdermal films actually served as drug reservoirs.
The composite matrices are of different graded strengths and release properties. This is evident from the varied characteristics of the different batches. However, in applying these results, it is important to consider that the dermato-pharmacokinetics in both ex vivo and in vivo models may differ.
Click to view
Click to view
4 Conclusion
Polymeric materials and their composite mixtures were effectively used to develop transdermal diazepam patches/films for application on the skin. The products were stable and physicochemical and mechanical properties were satisfactory. Their behaviour with water was dependent on the composition. These patches maintained controlled release and permeation of diazepam across excised rat skin (ex vivo).
With the successful inclusion of diazepam in transdermal devices, rapid termination of therapy and less injurious care can be achieved.
Conflict of Interest
There is no conflict of interest.
References
[1] Kumar A, Pullankandam N, Prabhu S L, Gopal V. Transdermal drug delivery: an overview. Int. J Pharm Sci. Review Res 2010; 3(2): 49-54
[2] Divya A, Rao MK, Gnanprakash K, Sowjanya A, Vidyasagar N, Gobinath M. A review on current scenario of transdermal drug delivery system, Int J Res Pharm Sci 2012; 3(4): 494-502
[3] Chien YW. Drug delivery systems. Drugs and the pharmaceutical sciences, Vol.50, Marcel Dekker, New York, NY. 1992. Pp 797
[4] Amnuaikit C, Ikeuchi I, Ogawara K, Higaki K, Kimura T. Skin permeation of propranolol from polymeric film containing terpene enhancers for transdermal use. Int. J. Pharm. 2005; 1–2: 167–178
[5] Davis SS, Illum L, Tamlinson E. Enzymatic barriers to peptide and protein absorption and the use of penetration enhancers to modify its absorption. In: Lee VHL, editor. Delivery Systems for Peptide Drugs. New York: Plenum Press, Dekker; 1986. p.389–416.
[6] Wohlrab J, Kreft B, Tamke B. Skin tolerability of transdermal patches. Expert Opin Drug Deliv 2011; 8 (7): 939-948
[7] Fan LT, Singh SK. Controlled release: A quantitative treatment. New York: Springer-Verlag; 1989. pp 13-56, 85-129
[8] Julraht K, Keith AP, James AW. Development of a Transdermal Delivery Device for Melotoin: In-vitro Study. Drug Dev. Ind. Pharm 1995; 21: 1377–1387.
[9] Ho HO, Chen LC, Lin HM, Sheu MT. Penetration Enhancement by Menthol Combined with a Solubilization Effect in a Solvent System. J. Control. Rel 1998; 51: 301–311.
[10] Zhang H, Xia H, Zhao Y. Poly(vinyl alcohol) hydrogel can autonomously self-heal. ACS Macro Lett. 2012; 1: 1233–1236
[11] van Ravenzwaay B, Leibold E. A comparison between in vivo rat and human and in vivo rat skin absorption studies. Hum Exp Toxicol 2004; 23 (9): 421-430
[12] Russell LM, Guy RH. Measurement and prediction of the rate and extent of drug delivery into and through the skin. Expert Opin. Drug Deliv. 2009; 6 (4): 355-369