1.0 INTRODUCTION
Depression is a mental disorder that is associated with a gradual and nearly unnoticeable withdrawal from an active way of life and enjoyment of living of an individual [1]. It is caused by a combination of genetic, psychological, environmental and biological factors [2]. A causal relationship exists between depression and physical illness. People that are physically unwell have an increased risk of developing depression [3,4]. Depression worsens or even initiates cardiovascular [5, 6], cancer [7], Parkinson disease and diabetes mellitus [8]. The pathophysiology underlying the inter-relationship between depression and diabetes is complex and poorly understood [9,10]. The psychological stress, pains, decreased quality of life, complexity of management may contribute to the increased incidence of depression in diabetes. People with adult onset diabetes have a 25% chance of having depression and depression also affects about 70% of patients with diabetic complications [11,12,13,14].
The association between depression and decreased adherence to oral hypoglycaemic agent and the reported increase in the therapeutic scope of metformin beyond its antihyperglycemic effect have become a relevant clinical issue. For example, metformin has been reported to reduce risk of cancer [15,16] improve cognitive function [17] and has shown some therapeutic potential in Parkinson disease [18]. Long term use of metformin was found to remarkably decrease symptoms of depression in type 2 diabetic mellitus patients [19]. In spite of studies supporting the clinical efficacy of metformin in depression, there has been a considerable confusion between diabetes-related distress and depressive symptoms which stem from inadequate measurement that gives a clear distinction between clinical depression and nonpsychiatric emotional distress [20]. Based on literature report, metformin may possess antidepressant-like activity. This study therefore, aimed at investigating the antidepressant-like activity of metformin in acute models of depression.
2.0 MATERIALS AND METHODS
Drugs: Sertraline (Global Pharmaceuticals Ltd., England), metformin (Merck Pharmaceuticals Ltd., UK ) diazepam (ValiumR Roche, Switzerland).
Animals
Swiss Albino mice of both sexes weighing 18-25g were obtained from the Animal House Facility of the Department of Pharmacology and Toxicology, Igbinedion University Okada. The mice were kept in a propylene cage, fed with standard animal feed and allowed free access to water. Animals were acclimatised for five days before experimentation and the experiment was carried out between the hours 8:00 to 6:00 hours. Experimental protocols were followed according to the International standard for care and use of laboratory animals.
Antidepressant Study
Tail suspension test
The method described by Sterul et al [21] was adopted for the study. Thirty mice were randomly divided into five groups of six animals each. Group 1 received 10 mL/kg distilled water, group 2 received 10 mg/kg sertraline while group 3, 4 and 5 received 100, 200 and 400 mg/kg metformin respectively per oral. One hour after drug administration each mouse was suspended on the edge of the shelf 68 cm above a table top by an adhesive tape placed approximately 1 cm from the tail end for a period of six minutes. The immobility time defined as the time the animal hang passively and completely motionless was taken and recorded [21].
Forced Swim Test
The method described by Alpermann et al., [22] was adopted for the study. Thirty mice were randomly divided into five groups of six animals each. Group 1 received 10 mL/kg distilled water, group 2 received 10 mg/kg sertraline while group 3, 4 and 5 received 100, 200 and 400 mg/kg metformin respectively per oral. One hour after drug administration each mouse was placed in a plexiglass cylinder of 40 x 18 cm filled with water to the height of 15 cm at room temperature. Animals were allowed to swim for 5 minutes and immobility time defined as the time animal remains almost motionless with the head above the water was taken and recorded [22].
Exploratory Study
Open Field Test
The method described by Kalueff et al., [23] was adopted for the assessment of spontaneous locomotor (horizontal) and exploratory (vertical) activity. It consisted of plywood (72 x 72 x 36 x 36 cm), one of the walls is a clean transparent plexiglass for visibility. The base was divided into 16 squares (18 x 18 cm) with blue marker and covered with transparent plexiglass [24].
Thirty mice were randomly divided into five groups of six animals each. Group 1 received 10 mL/kg distil water, group 2 received 1.5 mg/kg diazepam while group 3, 4 and 5 received 100, 200 and 400 mg/kg metformin respectively per oral. One hour after drug administration each mouse was placed at the centre of the open field arena and allowed to explore for five minutes. The number of lines crossed and number of rearing were observed and recorded.
Statistical Analysis
Data were collected and analysed using One-way analysis of variance followed by Dunnett’s post-hoc test in statistical package for social sciences (SPSS).
3.0 RESULTS
Tail Suspension Test (TST)
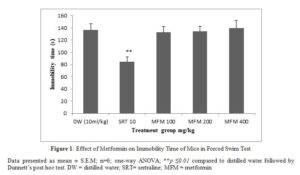
Metformin at all doses tested did not significantly alter the immobility time of mice compared to the distilled water group in the tail suspension test. Sertraline significantly (p ≤ 0.01) decreased immobility time (figure 1).
Forced Swim Test
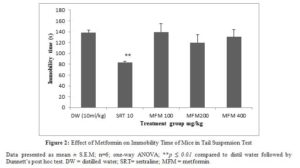
Metformin at all doses tested did not alter the immobility time of mice while sertraline significantly (p ≤ 0.01) decreased immobility time compared to the distilled water group (figure 2).
Open Field Test
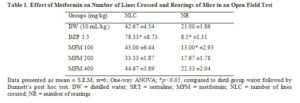
Metformin did not significantly alter the number of lines crossed at all doses tested. Number of rearing was significantly (p ≤ 0.05) decreased only at 100 mg/kg. Diazepam significantly (p ≤0.01) increased number of lines crossed and significantly (p ≤ 0.05) decreased number of rearing in the open field test (Table 1).
Click to view
Click to view
Click to view
4.0 Discussion
The inter-relationship between depression and diabetes is well reported. However, the pathophysiology underlying this inter-relationship is poorly understood [25, 26]. This has led to a considerable confusion between diabetes-related emotional distress and depressive symptoms which also extend to metformin improving depressive symptoms among diabetic patients [19, 14]. The proposed antidepressant-like effect of metformin is yet to be validated thus necessitates this study.
In the forced swim test and tail suspension test metformin could not decrease the immobility time compared to the control which implies the lack of antidepressant activity. Sertraline significantly decreased the immobility time in tail suspension test and forced swim test. The tail suspension and forced swim tests are acute behavior despair model of depression, employed in rodents to predict antidepressant potential of drugs by decreasing immobility time [27]. Immobility time of mice subjected to an inescapable stress in tail suspension and forced swim test represent a state of despair or hopelessness typical of depressive symptom in human [28,29]. Antidepressants and newer compounds with potential antidepressant activity decrease the immobility time in these tests. This can be correlated to an improvement in the state of despair in clinical depression.
The lack of antidepressant-like activity of metformin in the acute models of depression may suggest need for clear distinction between depressive symptoms and nonpsychiatric emotional distress in diabetes. This may have led to the claim of metformin improving depressive symptoms among diabetic patients. It is therefore possible that the improvement in depressive symptoms among diabetic patients using metformin may result from proper diabetic control that consequently improved diabetic emotional distress. Almost all available antidepressant act through the monoamine neurotransmission and are able to decrease immobility time in tail suspension and forced swim test [30]. This may also imply that the acute models of depression is more specific for screening agents acting through monoaminergic system despite involvement of other pathways in the pathophysiology of depression and its causal relationship with diabetes. The dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is hypothesised to be a biological association between depression and diabetes [30]. The HPA is activated by a wide variety of stressful stimuli with resultant increase in cortisol level, autonomic activity and activation of the proinflammatory cytokines. These all lead to diabetes mellitus and depression through alteration in the normal physiological processes [31].
Drugs with psychostimulants and central nervous system depressants activity can affect the immobility time thereby creating a false positive or negative effect. The open field test was employed to rule out this effect. In the open field test, metformin could not significantly alter the number of lines crossed which implies lack of stimulating or sedating effect. Diazepam significantly increased the number of lines crossed due to its anxiolytic effect. Metformin could not significantly alter the number of rearings except at 100 where it significantly decreased the number of rearings. The inability of metformin to alter the number of rearings may be due to lack of central nervous depressant activity. Diazepam significantly decreased the number of rearings due to its anxiolytic effect.
Conclusion
Metformin possesses no antidepressant-like activity in acute models of depression suggesting that it may not be acting through monoaminergic neurotransmission. The reported clinical improvement in depressive symptoms may result from an improvement in diabetic control possibly via other mechanism.
Acknowledgement
The authors are grateful to the entire management of the Department of Pharmacology and Therapeutics Igbinedion University, Okada for providing the necessary environment and equipments for this research.
Conflict of Interest
There is no conflict of interest
References
- de Zwart PL, Jeronimus BF, de Jonge P, et al. Emperical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: a systematic review. Epidemiology and Psychiatric Sciences. 2019; 28(5): 544-562. doi:10.1017/S2045796018000227.
- Radu V. Saveanu RV and Nemeroff BC. Etiology of Depression: Genetic and Environmental Psychiatr Clin N Am. 2012; 35: 51-71. doi:10.1016/j.psc.2011.12.001
- Hansen MS, Fink P, Frydenberg M, Oxhoj M, Sondergaard L, Munk-Jorgensen P. Mental disorders among internal medical inpatients: prevalence, detection and treatment status. J Psychosom Res. 2001; 50(4): 199-204
- Rayner L, Price A, Evans A, Valsraj K, Higgison IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev. 2010: (3): CDOO7503, DOI(3): CD007503
- Musselman D.L., Evans D.L., Nemeroff C.B. The relationship of depression to cardiovascular disease, epidemiology, biology, and treatment. Archives of General Psychiatry 1998; 55: 580-592
- Neu P. Correlation of depression with stroke. Pathophysiological mechanisms. Nervenarzt 2009; 80: 772
- Lazure K. et al.: Association between depression and survival or disease recurrence in patients with head and neck cancer enrolled in a depression prevention trial. Head Neck-Journal for Sciences Specialties Head Neck 2009; 31: 888-892.
- Peyrot, M. Depression: a quiet killer by any name. Diabetes Care 2003; 26: 2952-2953
- Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract. 2013; 99(2):98–104
- Tabák AG, Akbaraly TN, Batty GD, Kivimäki M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol. 2014; 2(3):236–2452
- Lamberg, L. Treating Depression in Medical Conditions May Improve Quality of Life. JAMA 1996; 276: 857-858.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24: 1069 –1078
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23:934–942
- De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosomatic Medicine 2001;63:619–630
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304-1305. 10.1136/bmj.38415.708634.F7
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC: Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011; 11: 20-10.1186/1471-2407-11-20
- Hsu CC, Wahlqvist ML, Lee MS, Tsai HN: Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis. 2011; 24: 485-493
- Wahlqvist ML, Lee MS, Hsu CC, Chuang SY, Lee JT, Tsai HN: Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord. 2012; 18: 753-758. 10.1016/j.parkreldis.2012.03.010
- Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41(9):650–656.
- Gonzalez JS, Fisher L, Polonsky WH. Depression in Diabetes: Have We Been Missing Something Important? Diabetes care 2011; 34(1).
- Steru L, Chermat R, Thierry B. and Simon P. Tail Suspension test: a new method for screening antidepressants in mice. Psychopharmacology 1985; 85: 367-370.
- Alpermann HG, Schaut U, Usinger P. and Hock FJ. Pharmacological effects of Hoc 249: A new potential antidepressant. Drug Development Research 1992; 25: 267-282.
- Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Tuohimaa P. Temporal stability of novelty exploration in mice exposed to different open field tests. Behaviour Processes 2006; 72(1): 104–112. doi:1016/j.beproc.2005.12.011.
- Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behavior Genetics 1999; 26: 263-271.
- Clarke DM, Currie KC, (2009) Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence J. 2009; 190(7): 54-60.
- Lin EH, Heckbert SR, Rutter CM, Katon WJ, Ciechanowski P, Ludman EJ, et al. depression and increased mortality in diabetes: unexpected cause of death. Ann Fam Med 2009 (5): 414-421
- Huwiler A. Tail suspension test. In: Vogel HG, editor. Drug Discovery and Evaluation: Pharmacological Assays. 3rd ed, Vol. 1. Springer-Verlag: Berlin, New York; 2008. p. 791-3
- Everton R, Cristiane QS, Eneas SFM, Ademar AFJ, Bruno SM, Jose GP. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. Journal of Ethnopharmacology 2018; 212: 43-49.
- Kashani L, Esalatmanesh S, Eftekhari F, Salimi S, Foroughifar T, Etasam F, Safiaghdam H, Moazen-Zaden E and Akhondzadeh S. Efficacy of Crocus sativus (saffron) in treatment of major depressive disorder associated with post-menopausal hot flashes: a double-blind, randomized, placebo-controlled trial. Archives of Gynecology and Obstetrics 2018; 1-8.
- Krishnan V and Nestler EJ. Animal Models of Depression: Molecular Perspectives. Curr Top Behav Neurosci. 2011; 7:121-147. doi: 10.1007/7854 2010 108
- Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological Basis of Depression in Adults with Diabetes. Curr. Diab. Rep. 2010; 10:396–405
- Joseph JJ and Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus Ann. N.Y. Acad. Sci. 2017; 20-34. ISSN 0077-8923
