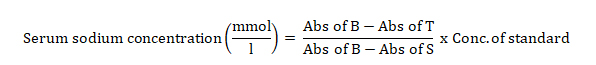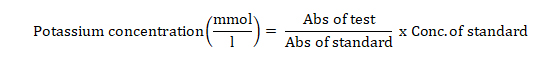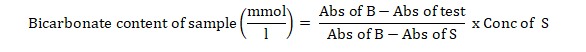1. INTRODUCTION
MSG has a diverse effect in animals [1] and adversely affected locomotor activities [2]. Adverse effect of MSG appears to manifest in MSG-sensitive individuals [3]. Lately, there is a shift from conventional and synthetic drug to natural products like medicinal plants, in a quest to provide alternative drugs in alleviating various disease conditions. Medicinal plants are defined as those plants capable of producing active ingredients that treat several diseases and hence achieving an important role in global health [4]. The utilization of plants products and its waste has shown great potential for new drugs development and correction of disease conditions [5]. Most of these plant wastes are disposed in such a way that they become source of environmental pollution [6].
Vernonia amygadalina commonly called ‘bitter leaf’ because of its bitter taste is a member of the Asteraese family, ethno-medically consumed either as a vegetable or aqueous extract as tonics for the treatment of various illnesses. Vernonia amygadalina produces a variety of flavonoids and bitter sesquitepene lactones which contribute to the bioactivities of this plant [7]. All parts of the plant are pharmacologically useful. Both the roots and leaves are used in phyto-medicine. In India, leaves, stem and root of Vernonia amygadalina are used in the treatment of HIV, measles, amoebiasis, influenza and mastitis infection [8].
Electrolytes are minerals in the body fluids that carry an electric charge. Electrolytes affect the amount of water, the acidity of blood (pH), muscle function and other important processes in the body [9]. Electrolytes are being regulated by the kidney, acid-base balance, insulin, aldosterone, gastrointestinal conditions and skin [10]. Administration of certain plant extracts such as Picralima nitida has been observed to tamper with the serum electrolytes levels [11].The use of synthetic chemicals as fungicides cause severe and long-term environmental pollution, acutely toxic, and can even cause cancer in humans and other animals. Fungicides of biological origin have been demonstrated to be specifically effective on target organisms and are also biodegradable. Biological control has become popular worldwide. Thus this study was aimed at determining the antifungal properties of Vernonia amygadalina stem extract and its effect on the electrolyte status of normal and monosodium glutamate-intoxicated rats.
2. MATERIALS AND METHODS
2.1 Materials
Chemicals: All the chemicals used were of analytical grade and were product of Sigma Aldrich, St. Louis, USA.
2.2 Plant Material and Extraction
Fresh stems of V. amygdalina were collected from Amawom Oboro, Ikwuano L.G.A in Abia State; the plant was identified and authenticated by Prof. H.O. Edeoga of the Department of Plant Science and Biotechnology, Michael Okpara University of Agriculture, Umudike. The dried stems were then taken for pulverization by a pulverizing machine in the Department of Soil Science, National Root Crops Research Institute, Umudike. A yield of 1.5 kg of the powder was obtained and 550 g of the powder was soaked in 2.2 litres of analyte ethanol for a period of 48 hours. The resulting mixture was then filtered using the Whatman’s filter paper. The filtrate obtained in a beaker of known weight was placed in a water bath at temperature of 40° C. After evaporation of the ethanol, the crude extract was refrigerated at 2- 8° C until use.
2.3 Experimental Animals
20 adult male albino rats (Ratus norregicus) which weighed between 120–160 g were procured from the animal house of the Department of Biochemistry, College of Natural Sciences, Michael Okpara University of Agriculture, Umudike. They had free access to pelleted feed (Vital Feed) and clean water as they were allowed to acclimatize for two weeks.
2.4 Experimental Design
The rats were divided into five groups of four each as follows:
Group A: Rats administered MSG only
Group B: Rats administered the extract only
Group C: Rats received only feed and clean tap water
Group D: Rats administered MSG and a low dose (200 mg/kg b.w.) of the extract
Group E: Rats administered MSG and a high dose (400 mg/kg b.w.) of the extract
Accurately weighed quantity of hydro-alcoholic (98 % ethanol) extract of V. amygdalina stem was suspended in distilled water and was administered orally to the experimental animals. MSG was freshly prepared in distilled water and administered orally with the aid of a gavage. Treatment was by daily intubation and lasted for 14 days.
2.5 Determination of Antifungal Activity:
Anti-fungal activity was evaluated using the agar well diffusion method as described by Magaldi et al. [12].
This was achieved by uniformly spreading 1 ml of fungi suspension prepared with sterile 0.85 % physiological saline solution on Saboureaud Chloramphenicol Actidione (SAC) plates. Afterwards, inoculums absorption SAC wells were made using sterile cock borers, which were then filled with 0.1 ml of the different concentrations Vernonia amygdalina stem extract was dissolved in distilled water (5 mg/ml). The control was carried out by filling the wells with 0.1 ml of mg/ml dissolved in sterile distilled water. The same procedure was followed for the determination of the diameters of inhibition of the antifungal agent ketonazole. Plates were incubated in steam room at 270 C. The results were read 5 days later. Standard diameter of inhibition was 25 ± 5. Every test was carried out in triplicate.
2.6 Determination of Serum Sodium Ion Concentration:
Serum sodium concentration was estimated using colorimetric method based on modified Maruna and Trinders method as described by Trinder [13].
To two test tubes labeled “test” and “standard” were added 1000 µl of precipitating reagent followed by the addition of sodium standard (10 µl) into the standard-labelled test tube and 10 µl of serum into the test-labelled test tube. The tubes were shaken vigorously and incubated for 5 minutes at room temperature and centrifuged at 2000 rpm for 2 min to obtain a clear supernatant. New test tubes; blank, standard and test were labelled and 1000 µl of sodium colour reagent was added to each of the tubes followed by the addition of standard supernatant (20 µl) into the standard-labelled tube and 20 µl of test supernatant obtained into the test-labelled tube; 20 µl of the precipitating reagent was added into the blank. The tubes were mixed and allowed to stand for 5 minutes at room temperature. The absorbance of standard and test against reagent blank was read and recorded
Where Abs = Absorbance
B = Blank, T = Test and S = standard.
2.7 Determination of Serum Potassium Ion Concentration
Serum potassium ion (K+) concentration was determined using the turbidometric method as described by Henry et al. [14].
Into two test tubes labelled standard and test were added 1000 µl of potassium reagent followed by the addition of standard (25 µl) into the standard-labelled tube and 25 µl of serum into the test-labelled tube. The tubes were shaken and incubated for 5 min at room temperature. Spectrophotometric reading was taken at 578 nm within 10 minutes. Serum potassium concentration was calculated as thus:
2.8 Determination of Serum Calcium Ion Concentration
Serum calcium concentration was determined using the colorimetric method as described by Faulker and Meites [15].
Working reagent (1.0 ml) was added into test tubes labelled standard, control and test. Again, there was addition of 0.02 ml (10 µl) of samples to the respective tubes. The content was thoroughly mixed and allowed to stand for 60 sec at room temperature. The spectrophotometer was zeroed with blank at 570 nm and the absorbances of all tubes were read.
2.9 Determination of Serum Bicarbonate Ion Concentration
Serum bicarbonate ion was determined using enzyme spectrophotometric procedures as described by Forrester et al. [16].
To test tubes labelled blank, standard, control, and test were added 1.0 ml of CO2 reagent and incubated for 3 minutes at 37o C. The spectrophotometer was zeroed with blank at 340 nm and maintained at 37o C. Water (0.005 ml) was pipette into the cuvettes labelled blank, standard and test respectively, followed by mixing via inversion and incubated for 5 minutes. The absorbances of all cuvettes were read at 340 nm.
2.10 Method of Statistical Analysis
The results were expressed as Mean ± Standard Error of Mean (SEM) of replicate determinations and were subjected to one way analysis of variance (ANOVA), using Statistical Package for Social Sciences (SPSS-20) at 95 % level of confidence. Values were considered statistically significant at p < 0.05.
3. RESULTS
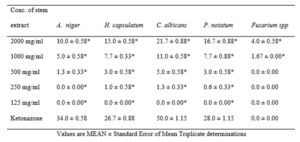
Data presented in table 1 below shows the antifungal activity of the stem extract of Vernonia amygdalina when tested separately against the five test human pathogens. A. niger inhibition by varying doses of the extract were significantly (*p < 0.05) lower than antifungal activity of ketonazone. Inhibition of H. capsulatum by doses of the extract were significantly (*p < 0.05) lower than antifungal activity of ketonazone. Ketonazone showed higher significant (*p < 0.05) inhibition of C. albicans compared to graded doses of the stem extract. P. notatum inhibition by graded doses of the extract were significantly (*p < 0.05) lower than antifungal activity of ketonazone. Inhibition of Fusarium spp (4.0 ± 0.60) at 2000 mg/ml of extract Vernonia amygdalina stem showed a significant (*p < 0.05) increase compared to ketonazone.
Click to view
Results in table 2 below show that there was no significant (*p < 0.05) difference in the bicarbonate levels between all the groups tested and the Normal control group. There was a significant (*p < 0.05) increase in potassium ion in all groups; MSG, extract, MSG+ Extract of Vernonia amygdalina stem (200 mg/kg and MSG+Extract of Vernonia amygdalina stem 400 mg/kg) (27.7 ± 0.50, 28.1 ± 0.60, 27.4 ± 0.50 and 28.4 ± 0.50) compared to normal control group (29.0 ± 0.80).The sodium level in the normal saline group (124.6 ± 2.70) was significantly (*p < 0.05) higher than MSG, Extract, MSG+Extract of Vernonia amygdalina stem (185.5 ± 1.40, 177.3 ± 1.20, 185.3 ± 1.70 and 149.5 ±0.70). There was a significant (*p < 0.05) increase in the calcium levels of the MSG group, Extract group and MSG+ Extract of Vernonia amygdalina stem 200 mg/kg (6.4 ± 0.50, 5.5 ± 0.05, 6.4 ± 0.80 and 4.6 ± 0.10) compared to group of normal saline (4.6 ± 0.10). The MSG+ Extract (400 mg/kg) was however non-significant (*p > 0.05) compared to the normal group.
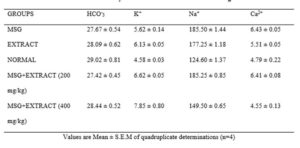
Click to view
4. DISCUSSION
Results obtained during assay with the extract of Vernonia amygdalina stem showed its inhibitory effect at all concentrations against the five pathogenic fungi. All concentrations of the extract effectively suppressed the mycelial growth of these fungi and this effect was found to be dose-dependent. In a previous work by Fadina [17], ethanol and aqueous extracts of this plant at various concentrations inhibited the mycelial growth of the fungus Colletotrichum lindemuthianum. Two sesquiterpene lactones (vernolide and vernodalol) isolated from the leaves of V. amygdalina were found to inhibit the mycelial growth of some fungi [18].
Extract of Vernonia amygdalina stem caused decreased serum Na+. There was no significant difference in serum Na+ levels after administration of the ethanolic extract of Vernonia amygdalina stem and MSG. Even though the Na+ level increased significantly (*p < 0.05) at a low dose (200 mg/kg), it decrease at higher dose (400 mg/kg) compared to the control. This implies that the extract V. amygdalina stem possesses antihypertensive effect at a higher dose. The extract showed a significant effect on the calcium levels of the test animals compared to the control. It was observed that at 200 mg/kg b.w. of V. amygdalina extract, the calcium level was significantly (*p < 0.05) reduced while at 400 mg/kg b.w., it was significantly (*p < 0.05) increased close to that of control; indication that a higher dose adversely alters the calcium electrolyte balance in the body. There was no significant (*p < 0.05) difference between the serum bicarbonate levels of the test groups compared to the control group. The ethanol stem extract of Vernonia amygdalina showed a significant effect on the potassium levels of the test animals as compared with control. It was observed that at 200 and 400 mg/kg b.w. of V. amygdalina extract, the potassium levels were significantly (*p < 0.05) compared to the control. This suggests a probable impairment of the kidney functionality due to administration of the extract.
5. CONCLUSION
The results of present study validated the traditional usage of the stem extract of Vernonia amygdalina against fungal infection. The extract of V. amygdalina stem did not have significant adverse effect on the functions of the kidney and maybe other organs of the body though the lowest dose caused significant perturbations in few serum electrolytes. Thus, the stem extract is a potential antifungal agent to be exploited by the pharmaceutical industry in the development of new antifungal drug with little or no adverse effects.
ACKNOWLEDGEMENT
We wish to acknowledge the staff members of Departments of Biochemistry and Plant Science and Biotechnology, Michael Okpara University of Agriculture, Umudike for their assistance during the period of this study.`
REFERENCES
[1] Egbuonu ACC, Obidoa O, Ezeokonkwo XA, Ejikeme PM, Ezeanyika, LUS. Some biochemical effects of sub-acute oral administration of L-arginine on monosodium glutamate fed Wistar albino rats 1: Body weight changes, serum cholesterol, creatine, and sodium ion concentrations. Toxicol Environ Chem 2010; 92(7): 1331-1337.
[2] Eweka AO, Om’iniabohs FAE. The Effects of Monosodium glutamate on the open field locomotor activities in adult Wistar rats. Int J Nut Wellness 2008; 6(2).
[3] Egbuonu ACC, Osakwe ON. Effects of high monosodium glutamate on some serum markers of lipid status in male Wistar rats. J Med & Med Sci 2011; 2(1): 653-656.
[4] Lima XF, Fenades-Ferreita M, Preiva-Wilson C. Pherolic Compounds protect Hep G2 cell from Oxidative Damage Relevance of Glutathione levels. L. Fe Sci 2006; 79: 2056-2068.
[5] Maryam F, Mohammed EB. Evaluation of physicochemical properties of Iranian mango seed bernel oil, 2nd International Conference on Nutrition and Food Sciences IPCBCE 2013: 53-59.
[6] Maisulthisakul P, Pasuk S, Rithiruangeles P. Antioxidant properties of phenolic phytochemicals from various cultivars of Thai mango seed kernels, Asian J Food and Agro-ind 2008; 1: 87-96.
[7] Favi F, Cantrell CL, Mebrahtu T, Kraemer ME. Leaf peltate glandular trichomes of Vernonia galamensis ssp. Galamensis vernonia, Ethiofica gilbert: Development, utrastructure, and chemical composition. Int J Plant Sci 2008; 169: 605-614.
[8] Swee KY, Wan YH, Boon KB, Woon SL, Huynh K, Abdul HN, Neo-jahan BA. Vernonia amygadalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bio-activities. J Med Plants Res 2010; 4(25): 2787-2812.
[9] Hills J. Management of IV Fluids and electrolytes centre for Rural health (Rm, BSN, MSN (cancer), SpecertCR(Onc) 2013.
[10] Ilegbedion IG, Onyide FM, Digba KA. Evaluation of MSG on Electrolyte Balance and Histology of Gastroesophageal Mucosa. Middle-East J Sci Res 2013; 18(2): 163-167.
[11] Nwankwo NE, Egbuonu ACC, Nduka FO, Nwodo OFC. Effect of seed extract of Picralima nitida on haematological parameters of malaria-infected albino mice and its interference with the serum electrolyte levels. Ife J Sci 2017; 19(2): 379-388.
[12] Magaldi S, Meta-Essayag S, Hartung de Capriles C, Perez C, Colella MT, Olaizola C, Ontiveros, Y. (2004). Well diffusion for antifungal susceptibility testing. Int J Infect Dis 2004; 8: 39-45.
[13] Trinder P. A rapid method for the determination of sodium in serum. Analyst 1951; 76: 596.
[14] Henry RF, Cannon DC, Winkelman JW. Clinical chemistry principals and techniques, 2nd Ed. Harper, and Roe, Hagerstown, MD 1974.
[15] Faulker WR, Meister S. Selected Methods for the Small Clinical Chemistry, Washington D.C. 1982; 125 p.
[16] Forrester RL, Wataji LJ, Silverman DA, Pierre KJ. Enzymatic method for determination of CO2 in serum. Clin Chem 1976; 22: 243-245.
[17] Fadina OO. The antifungal and nematicidal potentials of Vernonia amygdalina (Dal).Acta Horticul 2010; 853: 357-362.
[18] Erasto P, Grierson DS, Afolayan AJ. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacol 2006; 106(1): 117-120.