1.0 INTRODUCTION
Plant medicine has been used by Most of the world’s population due to its medicinal values and scarcity [1]. Medicinal plants have been used to cure a wide range of illnesses since ancient times [2]. Despite recent technological advancements in modern medicine, 75% of African communities still rely on traditional medicinal herbs for everyday healthcare. Many ailments can be treated using medicinal plants alone or in conjunction with other plants. Plants contain secondary metabolite chemicals, or non-nutritive compounds, called phytochemicals, which have the ability to prevent or treat disease. These nutrients are non-essential since the human body does not need them to survive. These compounds are produced by plants as self-defense, but new studies show that they can also serve as disease defense [3]. Bitter leaf, or Vernonia amygdalina Del., is a medicinal plant whose fresh leaves are very important to a human diet since they contain vitamin and mineral salts [4]. It is significant that Vernonia amygdalina has been utilized by humans for sustenance, maintenance, illness prevention, and treatment. Review of the literature has been discovered that the plant, particularly the leaf, is helpful in the ethnotherapy of diabetes [5], asthma [6], skin infections like ringworm, rashes, and eczema, schistosomiasis, malaria [7], measles, diarrhea, tuberculosis, abdominal pain, and intestine complaints, in addition to fevers, coughs, inducing fertility in women who are barren, and hyperlipidemia [8]. Properties of extracts have been used as an anthelmintic, antimalarial, anti-tumorigenic, bacteriostatic, and bactericidal action on some bacteria [9]. In particular, [5] documented the leaf extracts possess hypoglycemic and hypolipidemic effects. Several investigations have verified that V. amygdalina contains a variety of bioactive substances, including flavonoids, saponins, alkaloids, tannins, phenolics, terpenes, steroidal glycosides, triterpenoids, and various forms of sesquiterpene lactones [10;11]. The aim of this study is to investigate the effect of drying on phytochemicals and antioxidant property of Vernonia amygdalina leaves.
2. Materials and Methods
Study Area
This study was carried out at the Department of Biochemistry, Bayero University Kano, Nigeria
Sample Collection
The plant leaves were collected at school of continuous studies of Bayero University Kano, Kano State of Nigeria and identified at Department of Plant Biology Bayero University Kano with the following BUKHAN number 143 for future reference. The plant leaves were washed and divided into two part for drying methods sun-dried, shade-dried and fresh leaves for 15days. The dried samples were pulverized into fine powder, while the fresh leaves were crushed, both the samples were weighed using electric weighing balance Elite 210-4, kilotech.
Extraction of the plant leaves
Aqueous methanol extract of the powder sample was prepared by soaking 100 g of the sun dried, shade dried and fresh powdered leaves in 750 ml of absolute methanol and 1500 ml of distilled water (1:2) at room temperature for 72 hrs. The aqueous methanol extract was filtered and subsequently passed through cotton wool and concentrated using a rotary evaporator with the water bath set at 40oC to one-tenth its original volume and finally with a freeze drier. The dried residue was then stored at 4ºC. Portions of the extract was weighed and dissolved in distilled water for experimental analysis.
Phytochemical Screening
Determination of Total Phenolic Content
The weight of each aqueous methanol extract sample was measured (0.01 g) and then they were dissolved in 10 ml of distilled water. 1 ml of the solution was moved into a test tube, then 0.5 ml of (2N) folin-ciocalteu reagent and 1.5 ml (20%) Na2CO3 solutions were then added. The volume was raised to 8 ml with distilled water, vigorously shaken, left for 2 hours, and the absorbance was measured at 765 nm. The total phenolic content of the extract was determined by using a standard calibration curve with ascorbic acid solution and expressed in mg of ascorbic acid per gram of dry weight [12]. The phenolic content concentrations of the three extracts were determined using the ascorbic acid standard curve equation.
Y=0.0014x
Where y= absorbance
x=concentration
Determination of Total Flavonoids
Each leaf extract (0.01 g) weight was measured and then it was dissolved in methanol. After that, it was combined with 100 microliters of 20% aluminum trichloride and a drop of acetic acid before being thinned with methanol to 5 ml. An absorbance measurement at 415 nm was conducted following a 40-minute incubation period of the solution. A blank sample was created by mixing 100 ml of plant extract with a drop of acetic acid, followed by dilution to 5ml with methanol. The standard rutin solution (0.5 mg\ml) in methanol was measured for absorbance under identical conditions [13]. The flavonoids content in three extracts was determined by using the rutin curve equation as per standard procedure.
Y=0.0062x+0.0012
Determination of Total Alkaloids
0.01 g of every sample was dissolved in 5 ml of 2N HCl and then filtered. 1 ml of the filtrate was moved into a separatory funnel and rinsed with 10 ml of chloroform. 1 ml of the solution was moved to a separatory funnel, followed by the addition of 5 ml of bromocresol solution and 5 ml phosphate buffer. The combination was agitated and the complex formed was fractioned with chloroform through vigorous shaking. The sample was placed in a 10 ml flask and mixed with chloroform until it reached the brim. The measurement of the complex’s absorbance in chloroform was done at 470 nm [13]. Calculating the alkaloid concentrations of the three extracts was done using the standard aconitine curve equation.
Y=0.006x-0.003
Determination of Total Tannins
A 0.2 g sample was placed in a 50 ml plastic bottle, and then 20 ml of distilled water was added and shaken for an hour. The mixture was filtered in a 50 ml volumetric flask, topped up to the mark. 5 ml of the filtrate was pipetted into a test tube and mixed with 2 ml of 0.1M FeCl3 in 0.1N HCl and 0.008 potassium ferrocyanide. The solution was left to stand for 10 minutes before measuring the absorbance at 620 nm [13].
The concentration of the flavonoids contents of the three extracts were calculated following the standard gallic acid curve equation:
Y=0.0027x+0.0036
Determination of Total Saponins Content
10 ml of distilled water was mixed with 0.1 g of each extract, followed by the addition of 10 ml of vanillin and 25 ml of 72% sulphuric acid to the solution. The mixture was thoroughly stirred and placed in a water bath at 60°C for 10 minutes, then cooled in ice water. The absorbance at 544 nm was measured and expressed as diosgenin equivalents (mg/g DE extracts) [14].
The concentration of the flavonoids contents of the three extracts were calculated following the standard diosgenin curve equation:
Y=5.636x
ANTIOXIDANT ESTIMATION
Determination of Antioxidant capacity by Phosphomolybdenum method
0.1mL of extract solution (100 µg/mL, 50 µg/mL, and 25 µg/mL) was combined with 1mL of reagent solution (0.6M sulphuric acid, 30mM sodium phosphate, and 4mM ammonium molybdate) to create a mixed aliquot. The tubes containing samples were closed and placed in a water bath at 95°C for 90 minutes to incubate. Following incubation, the reactant samples were allowed to cool down to room temperature,
then the absorbance of each aqueous solution was measured at 695 nm in comparison to a blank. A standard blank included 1 mL reagent solution and an equal amount of solvent as the samples, and then it was incubated under identical conditions as the other samples [15].
Determination of Reducing power assay.
The aqueous methanolic leaf extract of Vernonia amygdalina was assessed for its reducing power capacity following the procedure outlined by Oyaizu [16]. The mixture had 1.0 mL of different concentrations of extracts (100 µg/mL, 50 µg/mL, and 25 µg/mL) and 2.5 mL of 0.2M sodium phosphate buffer. The blend was kept at a temperature of 50°C for half an hour, and the process was stopped by adding 2.5 mL of 10% trichloroacetic acid. After centrifuging at 3000r/min for 10 minutes, 2.5 mL of deionized water and 0.5 mL of 0.1% ferric chloride were included. The measurement of absorbance at 700 nm was done by comparing it to a blank solution consisting of deionized water and phosphate buffer. Ascorbic acid was employed as the standard. The findings were presented in terms of the amount of ascorbic acid equivalent per gram (AscAE/gm).
Hydrogen Peroxide Scavenging (H2O2) Assay.
The ability of extracts to scavenge hydrogen peroxide was estimated by following the method of [17]. The extract was dissolved in a phosphate buffer at a concentration of 1 mg/ml. A quantity of 1 ml of extract was added to 3.4 ml of phosphate buffer (5mM, pH 7.4) followed by 600 μl of 400 mM H2O2. The solution was kept at room temperature for 4 min and absorption was measured at 230 nm. Ascorbic acid was used as control. The percentage of hydrogen peroxide scavenging was calculated as follows:
Scavenged H2O2 (%) = [Ai − At]/Ai × 100
Where Ai was the absorbance of control and At was the absorbance of test samples.
3. RESULTS AND DISCUSSION
Result
Physical Properties of the Plant Extracts
Three extracts were obtained from V. Amygdalina leaves. The aqueous methanol leaf extract of the fresh plant was brownish in colour and gummy in texture. The aqueous methanol leaf extract of the sun-dried plant was black-red in colour and sticky in texture. The aqueous methanol leaf extract of the shade dried plant was also greenish in colour and sticky in texture. Weight of plant sample before and after extractions were obtained as shown in Table 1
Table 1: Physical Properties of Leaf Extracts from V. Amygdalina
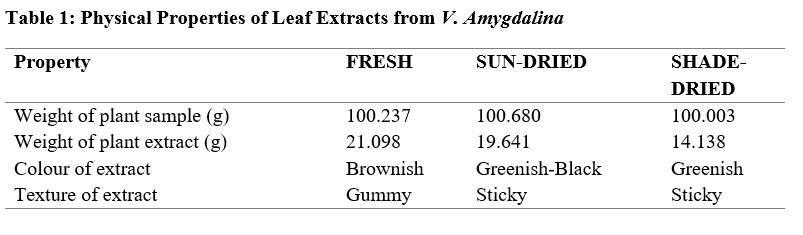
Table 2: Quantitative Phytochemical Analysis
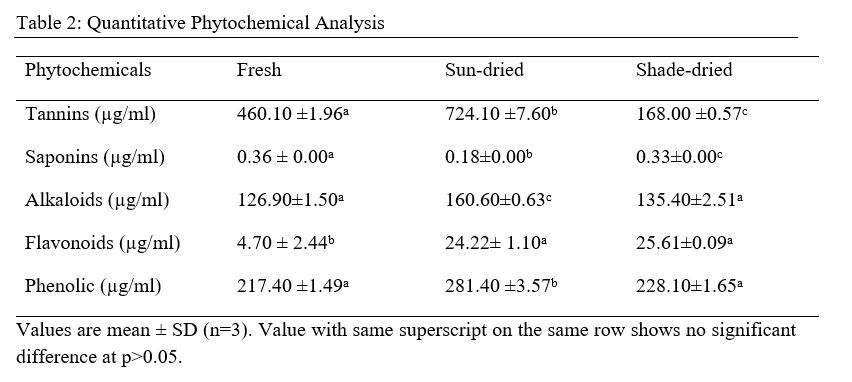
Table 3: Reducing Power Assay of Shade-Dried, Sun-Dried and Fresh Aqueous Methanol Leaf Extract of V. Amygdalina
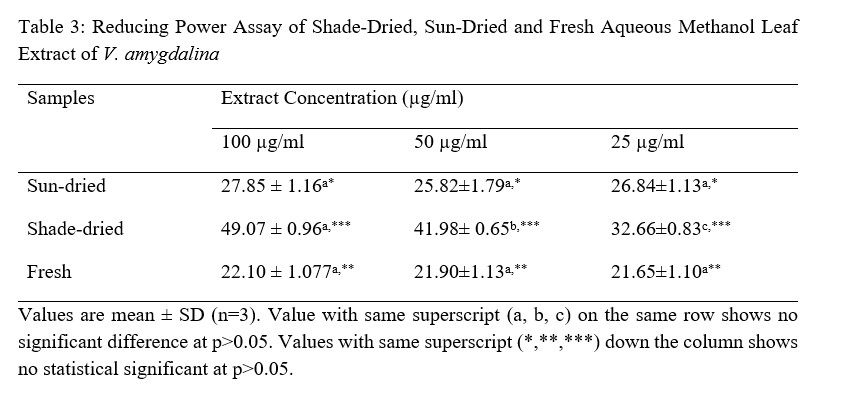
Table 4: Phosphomolybdate Assay extracts Concentration (µg/ml)
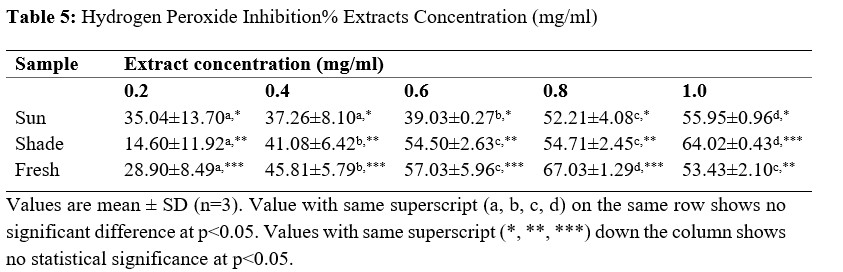
Table 5: Hydrogen Peroxide Inhibition% Extracts Concentration (mg/ml)
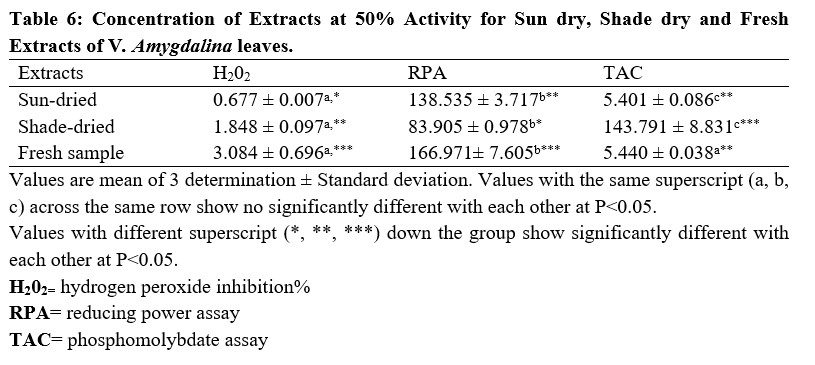
The result for the concentration of the extracts producing 50% Reducing Power Assay, Hydrogen Peroxide Inhibition% and Phosphomolybdate Assay (IC50) are shown in the Table 6 below.
Table 6: Concentration of Extracts at 50% Activity for Sun dry, Shade dry and Fresh Extracts of V. amygdalina leaves.
Discussion
From the results obtained from the phytochemical quantitative determination of the sun-dried, shade dried and fresh aqueous methanol leaf extract of V.amygdalina reveals that Tannins was the highest concentration of all the phytochemical screened among the three samples (fresh, sun and shade dried) with a value of 460.10 ±1.96, 724.10 ±7.60 and 168.00 ±0.57 respectively. This is followed by Phenolic with corresponding values of 217.40 ±1.49, 281.40 ±3.57 and 228.10±1.65 for fresh, sun-dried and shade-dried sample respectively. The least phytochemical present was saponins in all three sample (Table 4.2). The values of the phytochemicals are much higher in the Sun-dried samples when compared to the other two (Shade-dried and fresh) with a significant difference. This is then followed by shade-dried sample where phenolic, flavonoids, alkaloids and tannins were higher than those of fresh sample with values of 168.00 ±0.57, 135.40±2.51, 25.61±0.09 and 228.10±1.65 respectively. However, the Saponin was found to be highest in the Fresh sample (0.36 ± 0.00), then the shade-dried (0.33±0.00) and the least value was for Sun-dried (0.18±0.00). This result is in agreement with that of Misari [18] where, from his results, the Saponin level was found to be least while phenolic; flavonoid and tannin were the highest. In another work by [19], the Sun-dried sample contains higher amount of all the five phytochemicals screened as compared with the shade-dried. However, their result does not include values for fresh sample. From the results obtained from the Anti-oxidant property using the Reducing power assay (Table 4.3), it was observed for all the three samples, the values increases with an increase in the concentration of the extract used with 100 µg/ml having the highest values, then 50 µg/ml and the least values were recorded for plant extracts at 25µg/ml. Comparing the three extract samples it was found out that the values obtained for Shade-dried samples were the highest (49.07 ± 0.96, 41.98± 0.65 and 32.66±0.83) for the three (3) different concentrations respectively (Table 4.2). This is then followed by those of Sun-dried plant extracts and the least was recorded for fresh sample Mbakwem [20] also observed similar result from his study. He recorded that the Anti-oxidant property of V.amygdalina leaf extract using reducing power assay is concentration dependent as the highest values were recorded at a concentration of 250 µg/ml and the least at 50 µg/ml. Table 4.4 shows the result for the Phosphomolybdate assay (total anti-oxidant capacity) of the three plant extracts at different concentrations of 100 µg/ml, 50µg/ml and 25µg/ml. From the table, the highest activity was recorded for fresh plant extract at 100 µg/ml (717.6±3.12), followed by sun-dried extract at 50 µg/ml (703.7±9.45), sun-shade at 25 µg/ml (695.9±20.4). This result shows that the Phosphomolybdate activity of three extract of V.amygdalina is not concentration dependent. Table 4.5: shows the result for hydrogen peroxide %inhibition assay of the three plant extracts. The value for shade-dried samples increased steadily as the concentration increases from 14.60% (0.2mg/ml) to 54.02% (1.0mg/ml). The value of sun dried rise gradually and slowly as the concentration is increased from 0.2mg/ml till 0.6mg/ml from where the curve continues to rise sharply to attain a peak value of 55.45% at 1.0mg/ml. The fresh sample curve show a very rapid increase as the concentration of extract increased to obtain a peak value of 67.03% at the concentration of 0.8 mg/ml and then began to fall as the concentration increased to 1.0 mg/ml. The result shows that all the three extract have a very good ability as free radical scavenger and their ability is concentration dependence i.e. increased as the concentration increases. Comparison of the three extract (sun dried, shade dried and fresh sample) show that the highest peak was observed for fresh sample (67.03%) at 0.8mg/ml and the least from shade dried (14.60%) at 0.2 mg/ml. In Table 4.6, among the three extracts subjected to reducing power assay test in the study, it was observed that the fresh extract of V. Amygdalina leaves have the highest concentration with IC50 of 166.971 This is consecutively followed by Sun dried extract with IC50 of 138.535 and shade dried extract give the lowest concentration of IC50 of 83.905 . Hence comparing the sun-dried extract and fresh extract has less effective compared to shade-dried. On hydrogen peroxide inhibition percentage, the three extract show lowest concentration with IC50 on sun-dried (0.677) extract followed by shade dried extract with IC50 (1.848) and highest in fresh extract with IC50 (3.084. And on the phosphomolybdate assay shade dried extract record highest activities (143.791 ) followed by fresh extract (5.440) and sun dried extract was the least (5.401). For the combined IC50 of the three test (H2O2, RPA and TAC) for the antioxidant property of V. Amygdalina, it was observed that the sun-dried extract show least combined IC50 value of (0.677 ± 0.007) for H2O2 and (5.401 ± 0.086) for TAC. Lower value of IC50 indicates a higher antioxidant property hence sun-dried extract is more effective compared to the shade and fresh samples.
However, shade-dried samples had higher activity than the fresh samples with an overall highest IC50 value for the three (3) tests conducted.
4. CONCLUSION
It was concluded that, drying process has a profound effect on some of the phytochemicals of the leave extract of V. Amygdalina. Exposure to the sun-dried extract increases the amount of alkaloids, phenols and flavonoids, tannin compared to the fresh sample extract while saponnin
decreases in the sun-dried extract. Shade-dried extract showed increase in the alkaloids, phenol and flavonoids content compared to the fresh sample extract while tannin and saponnin content decreases in the shade.
Acknowledgement
My profound appreciate go to Dr. Salisu Abubakar of Environmental lab of biotechnology Department of SHESTCO; Mallam Kamaludeen Babagana of Department of Biochemistry of Bayero University, Kano for their assistance during the course of this research.
References
- Hudaib M, Mohammad M, Bustanji Y, Tayyen R, Yousef M, and Aburjaye M. (2008). “Ethnopharmacological survey of medicinal plants in Jordan, Mujid nature reserve and surrounding area”. Ethnopharmacol; 120: 63-71.
- Gajalaksmi S, Vijayalashmi S, and Devi-Rajeswari V. (2012). “Phytochemical pharmacological properties of Annona muricata International Journal.” A Review. Pharm pharmal science; 4(2): 3-6.
- Breslin A. (2017). “The Chemical Composition of Green Plants”. Sciencing, Leaf Group Fatty Acid Profile of the Leaves of Growing in South Africa. Vernonia amygdalina. Food Chemistry, 104, 636-642. doi.org/10.1016/j.foodchem.2006.12.013.
- Sobukola O. P. and Dairo OU. (2007). Modeling Drying Kinetics of Fever Leaves (Ocimum viride) in a Convective Hot Air Dryer. Nigerian Food Journal, 25, 145-153.
- Nwajo H.U, (2005). Efficacy of Aqueous Leaf Extract of on Plasma Vernonia amygdalina Lipoproteins and Oxidative Status in Diabetic Rat Model. Nigerian Journal of Physiological Sciences, 39-42.
- Akah P. A, Okoli C.O. and Nwafor S.V, (2002). Phyto-therapy in the Management of Diabetes Mellitus. Journal of Natural Remedies, 2, 59-65.
- Masaba S.C, (2000). The Antimalarial Activity of Some Traditional Medicinal Plants. Ethiopian Journal of Health Development, 13, 211-216.
- Adaramoye O.A, Akintayo O, Achem J. and Fafunso M, (2008). Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vas Health & Risk Management 4: 235-241.
- Izevbigie EB, Bryant JL, Walker A, (2004). A novel natural inhibitors of extracellular signal- regulated kinases and human breast cancer cell growth, Experimental Bio Med., 229:163-169.
- Farombi E.O, and Owoeye O, (2011). Antioxidant and chemopreventive properties of. Vernonia amygdalina Garcinia biflavonoidand. Int J. of Environ Res Pub Health 8: 2533 2555.
- Luo, X., Jiang, Y., Fronczek, F. R., Lin, C., Izevbigie, E. B., Lee, S. and Lee, K. S. 2017. Isolation and Structure Determination of a Sesquiterpene Lactone (Vernodalinol) from Vernonia amygdalina Extracts. Pharmaceutical Biology. 49(5).
12. Ganapaty S, Ramaiah M, Yasaswini K, Nuthakki V.K, and Harikrishnareddy D. (2012). “Quantitative phytochemical estimation and evaluation of hepatoprotective activity of methanolic extract of Dendrodium ovatum (L.) Kraenzl. Whole plant against CC14 induced hepatoxicity. Journal of pharmacognosy and phytochemistry, 2(3):113-118.
- Gracelin D.H.S, de Britto, A.J, and Kumar P.B.J.R, (2013). Qualitative and Quantitative Analysis of Phytochemicals in five Pteris Species. International Journal of Pharmacy and Pharmaceutical Sciences; 5(1): 105-107.
- Satyanarayana, M.V., Satydev, TNVSS. Ssailaja, V. and Madhu, M. (2016). “Qualitative Phytochemical analysis of selected medicinal plant species by using various organic solvents. Journal of pharmacognosy and phytochemistry; 5 (2):25-29.
- Prieto P, Pineda M. and Anguilar M. (1999). Spectrophotometric quantitation of antioxidant Capacity through the formation of a Phosphomolybdenum Complex: Specific application to the Determination of Vitamin E. Anal. Biochem; 269: 337-341.
- Oyaizu M. (1986). Studies on products of browning reaction: antioxidative activities of browning reaction prepared from glucosamine. J. Nutr; 44 (6):307–15.
- Ruch RJ, Cheng SJ, and Klaunig, JE, (1989). Prevention of cytotoxicity and inhibition of Intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis; 10 (6):1003–1008.
- Misari S. M, (1992). Further observation on the insects attacking bitter leaf in Samaru, Northern Nigeria. Savannah; 13(1): 1-13.
19. Ola S.S, Catia G, Marzia I, Francesco V. F, Afolabi A. A. and Nadia M. (2009). HPLC/DAD/MS characterization and analysis of flavonoids and cynnamoil derivatives in four Nigerian green leafy vegetables. Food Chemistry; 115: 1568 -1574. http://dx.doi.org/10.1016/j.foodchem.2009.02.013.
- Mbakwem. Aniebo, C., Okoyomo, E.P., Ogugbue, C.J. and Okonko, I.O. (2012). Effects of Jatropha curcas leaves on common Dermatophytes and causative agent of Pityriasis versicolor in Rivers State, Nigeria. Nature and Science 2012; 10(12): 151-157.
