INTRODUCTION
Malaria remains a devastating global health problem associated with high mortality and morbidity most especially in developing countries. In 2019, malaria was reported to cause an estimated 229 million clinical cases and 409,000 deaths globally with more than 90% of the cases and deaths occurring in Africa [1]. Despite numerous control and prevention strategies including the use of insecticide-treated bed nets, artemisinin-based combination treatments, and indoor residual spraying interventions, malaria accounted for about 50% of out-patient visits, 40% of hospital admissions and 30% of mortalities in Nigeria [2]. The development and increase in appearance of drug-resistant parasites, insecticide-resistant Anopheles, absence of a malaria vaccine, conflicts that displaces people and rapidly raising circulation of fake antimalarial drugs has further
contributed to malaria mortality and morbidity [3]. New and effective drugs are thus necessary to fight this deadly disease.
The contribution of oxidative stress to the pathophysiology of malaria has been long established particularly in malaria-induced anemia and pathological changes in some organs in the body [4]. Recent findings have shown that compounds and medicinal plants with antioxidant activity may prevent the advancement of malarial infection and probably prevent its sequelae [5, 6-8]. Uapaca togoensis which belongs to the family Euphorbiaceae, is an evergreen shrub that has been used traditionally to cure several ailments [9-12]. Various parts of the plant have been documented to possess antibacterial, larvicidal, antifugal, antimicrobial activities, cytotoxic, analgesic, anti-inflammatory acctivites, antiplasmodial, antipyreitic and antioxidant activities [13-21]. The present study was conducted to evaluate the effects of fractions of U. togoensis on antioxidant and lipid peroxidation levels in serum of experimental mice infected with P. berghei malaria and to identify the most effective fraction.
MATERIALS AND METHODS
Chemicals and Reagents
Chloroquine, Methanol, Hexane, Ethyl acetate and Butanol (Sigma -Aldrich Company, USA),
Plant Collection
The plant was collected from Edumoga District, Okpokwu Local Government Area, Benue State, Nigeria. The plant was then identified and authenticated at the Department of Botany, Ahmadu Bello University, Zaria and voucher specimen number 1279 was obtained for future reference.
Plant Extraction and Fractionation
The stem bark obtained from the tree was then cleaned, cut into small pieces and dried in the laboratory at room temperature till a constant weight was obtained. The dried stem bark was pulverized and 2 kg of the powdered stem bark was weighed and soaked in 70% aqueous methanol for 3 days at room temperature with intermittent and frequent agitation in a clean maceration jar. The solution was filtered and the filtrate was kept in an evaporating dish to evaporate to dryness. The crude methanol extract was then fractionated using solvents of varying polarity (hexane, ethyl acetate, butanol).
To obtain the other fractions, 200 g of crude methanol extract (CME) was suspended in distilled water (500 mL). This solution was then sequentially partitioned with n-hexane, ethyl acetate and butanol in a separating funnel and yielded hexane (HFUT), ethyl acetate (EFUT), butanol (BFUT) and residual aqueous (RFUT) fractions. The hexane fraction obtained was very little and insufficient to carry out further studies. The other fractions were collected, dried, and stored in separate labelled air tight jars placed in a desiccator.
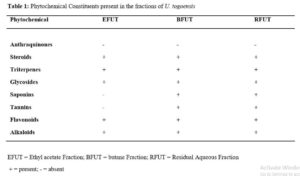
Phytochemical Screening
Phytochemical screening of the fractions was conducted to detect for the presence or absence of secondary metabolites using the method as described by Trease and Evans [22].
Inoculation of Plasmodium berghei NK65 Parasite
The parasite strain (Plasmodium berghei NK 65) used in the study was obtained from the Nigerian Institute of Medical Research (NIMR), Lagos, Nigeria. The parasites were maintained by constant intraperitoneal injection of 0.2 mL of infected erythrocytes containing about 1 X 107 P. berghei parasites into new groups of mice every 5-10 days. Experimental mice were inoculated intraperitoneally with 0.2 mL of infected blood containing about 1 x 107 P. berghei parasitized erythrocytes [23].
Experimental Animals
Swiss albino mice of both sexes weighing between 18–22 g was used for the study. The mice were gotten from the the Animal House Facility of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria. They were kept under standard laboratory conditions and fed with commercial pellet diet (Vital feed®, Nigeria) and normal water ad libitum. The experimental procedures involving animals were conducted in accordance with the criteria outlined in the Guide for the Care
and Use of Laboratory Animals by the National Institute of Health [24]. The study protocol was approved by the the Ahmadu Bello University, Zaria Committee on Animal Use and Care.
Oral Acute Toxicity Study
The median lethal dose (LD50) of each fraction was estimated in mice through the oral route using the method as described by Lorke [25]. Thirteen (13) mice were used for both the first and second phases of the experiment. For the initial phase, nine (9) mice were randomLy divided into three groups of 3 mice each and orally administered with the ethyl acetate fraction at doses of 10, 100 and 1000 mg/kg, respectively. Mice were observed for signs of toxicity and death every hour for the first 4 hours and then from time to time for 24 hours. In the second phase, 4 mice were orally administered with the fraction at doses that were determined based on the results from the first phase. The first animal was orally administered with the ethyl acetate fraction at dose of 1200 mg/kg. The second, third and fourth animals recieved the fraction at doses of 1600, 2900 and 5000 mg/kg respectively all through the oral route. Observations for signs of toxicity and death was carried out as previously described above. The oral LD50 value was determined by calculating the geometric mean of the lowest dose that caused death and the highest dose for which the animal survived as follows:
The same procedure was used to estimate the oral LD50 values of the butanol and residual aqueous fractions.
In vitro Studies using 2,2-Diphenyl-1-picryl-hydraxyl (DPPH) Radical Scavenging Assay
The DPPH radical scavenging assay method [26] was used to evaluate the fractions for antioxidant activity. A 0.1 mM solution of DPPH in methanol was prepared by dissolving 39.4 g of DPPH in 1L of methanol and this served as the blank (control). Five serial dilutions (0.01, 0.02, 0.03, 0.04 and 0.05 mg/mL) of the fractions (EFUT, FBUT and RFUT) of the plant and ascorbic acid (standard antioxidant) were prepared using methanol as the diluent. One (1) mL of the prepared DPPH was added to 3 mL of the various serially diluted concentrations of the fractions and ascorbic acid and kept in the dark for 30 minutes. The absorbances of the various samples were measured at wavelength of 517 nm using a UV spectrophotometer. All tests were performed in triplicate. The radical scavenging activity was calculated as follows:
Radical scavenging activity (%) = [(absorbance of control – absorbance of test sample) / absorbance of control] x 100 (Equation 2)
The obtained percentages were plotted against the various concentrations for each sample and IC50 values were estimated by non-regression line analysis.
Effect of Fractions on Antioxidant Enzymes and Oxidative Stress Markers in P. berghei infected Mice
The study was carried out using sixty-six (66) mice of both sexes. Each mouse was infected by intraperitoneal injection with 0.2 mL of mice blood containing approximately 1 x107 parasitized erythrocytes. Three days after inoculation (72 hours), thin blood smears were made from a tail cut of each mouse, fixed for 5 minutes using methanol, stained with 10% Giemsa stain in Phosphate buffer, pH 7.2 and examined microscopically under oil immersion (x100) for assessment of parasitemia. Mice with established parasitemia were then divided into eleven groups of six animals each and treated as follows:
Group I = Distilled water 10 mL/ kg, Groups II, III and IV = 250, 500 and 1000 mg/kg EFUT, Groups V, VI and VII = 250, 500 and 1000 mg/kg BFUT, Groups VIII, IX and X = 250, 500 and 1000 mg/kg RFUT and Group XI = CQ 5 mg/kg.
The fractions were orally administered once daily for 4 consecutive days. On the seventh day, the mice were starved overnight and sacrificed under chloroform anesthesia and blood collected.
Animal sacrifice and collection of blood specimen
Blood samples were collected through cardiac pucture into sterile plain bottles and centrifuged for 15 minutes at room temperature using 2,500 rpm to obtain the serum. The obtained serum was used for the analysis of superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and malondialdehyde (MDA) levels.
Determination of Superoxide Activity
To determine the levels of superoxide dismutase, the method of Mishra and Fridovich (1972) was employed. To 0.5 mL of serum in a test tube, 1.5 mL of phosphate buffer pH 7.8 was added and mixed thoroughly followed by centrifugation for 5 minutes. The supernatant (0.2 mL) was transferred into another test tube where 2.5 mL of phosphate buffer and 0.5 mL of adrenaline (0.011 mL epinepherine) were added. The absorbance of the test tube at 480 nm wavelength was read at interval of 30 seconds for 3 minutes against the blank. SOD was calculated in units as the amount necessary to cause 50% inhibition of the oxidation of adrenaline to adrenochrome during one minute.
Determination of Catalase Activity
The method as described by Aebi’s [27] was employed to measure the level of catalase. To a test tube containing 2.8 mL of 50 mM potassium phosphate (buffer pH 7.0), 10 uL of serum was added. To initiate the reaction, 0.1 mL of freshly prepared 30 mM hydrogen peroxide (H202) was added and the decomposition rate of H202 was measured at 240 nm for 5 minutes on a spectrophotometer. One unit of CAT activity represented the amount of enzyme that destroyed 1 µmole H2O2/min and was calculated using the formula:
Activity (unit/mL enzyme) = (3.45 x dilution factor) / (t min x 0.1) (Equation 3)
Where:
3.45 represents the decomposition of 3.45 μmol of hydrogen peroxide in a 3.0 mL reaction mixture to produce a decrease in absorbance at 240 nm from 0.45 to 0.40 absorbance units
t min corresponds to the time in minutes required for absorbance at 240 nm to decrease from 0.45 to 0.40 absorbance units
0.1 represents the volume (mL)
Determination of Glutathione Activity
The method described by Ellman [28] was adopted for the determination of glutathione activity. To 150 µL of serum (in phosphate – saline buffer pH 7.4), 1.5 mL of 10% trichloroacetic acid was added in a tube and centrifuged at 1,500 g for 5 minutes. Ellman’s reagent (0.5 mL) was added to 1 mL of the supernatant and the mixture was treated with 3 mL of phosphate buffer (0.2M, pH 8.0) Absorbance was then read at 412 nm. The quantity of GSH was obtained from the graph of a GSH standard curve plotted by taking absorbance at 412 nm on the Y-axis and concentration on the X-axis and expressed as mg/dl [28].
Determination of Lipid Peroxidation
The method described by Ohkawa et al., [29] was employed to assess the levels of MDA in the serum. To 150 µL of the supernatant from the serum in a tube, 500 µL of deionized water was added followed 250 µL of 1.34% thiobarbituric acid. Equal volumes of 40% trichloroacetic acid was then added to all the tubes. The mixture was shaken and incubated for 30 minutes in a boiling water bath at a temperature > 90ͦC. The test tubes were left to stand to cool to room temperature and the amount of MDA formed was measured as the intensity of the pink-coloured complex formed spectrophotometrically at 532 nm. The MDA level was calculated using the formula as shown below:
MDA (nmol/L) = (absorbance x 100) / 1.56 (Equation 4)
RESULTS
Click to view
Acute Toxicity Studies on the Fractions of U. togoensis
There were no signs and symptoms of toxicity observed in the animals after oral administration of the fractions. Also, no mortality was recorded. The oral median lethal dose was thus estimated to be > 5, 000 mg/kg.
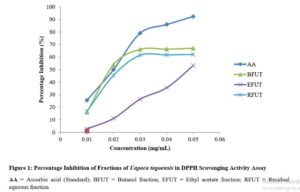
Radical Scavenging Activity of Fractions of Uapaca togoensis using DPPH assay
The results from the study showed that the fractions of Uapaca togoensis were able to scavenge the DPPH free radical to various extents. The radical scavenging effects produced were in the following order: AA>NFUT>RFUT>EFUT (Figure 1).
Click to view
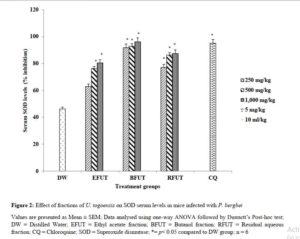
Effect of fractions of U. togoensis on superoxide dismutase (SOD) levels in serum of mice infected with P. berghei
There was a significant increase (p<0.05) in the SOD serum percentage levels produced by the ethyl acetate (82.85, 86.23 and 90.40 unit/mL), n-butanol (91.7, 92.82 and 96.1 unit/mL) and residual aqueous fractions (87.44, 86.23 and 90.4 unit/mL) compared to the negative control group as shown in Figure 2.
Click to view
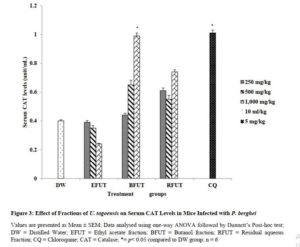
Effect of fractions of U. togoensis on catalase (CAT) levels in serum of mice infected with P. berghei
The butanol fraction produced a significant (p<0.05) increase in the level of serum catalase enzymes at only the 1000 mg/kg dose compared to the negative control group (treated with distilled water). The increase in serum catalase levels produced by the ethyl acetate and residual aqueous fractions were however insignificant at all the tested doses (Figure 3).
Click to view
Effect of fractions of U. togoensis on glutathione (GSH) levels in serum of mice infected with P. berghei
Administration of the fractions (ethyl acetate, n-butanol and residual aqueous fractions) of U. togoensis produced a significant (p<0.05) dose-dependent increase in serum GSH levels. However, the significant increase in serum GSH levels produced by the n-butanol and residual aqueous fractions at the 1000 mg/kg dose was more than that produced by chloroquine (5 mg/kg) the standard drug (Figure 4).
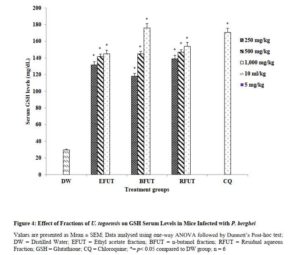
Click to view
Effect of fractions of U. togoensis on malondialdehyde (MDA) levels in serum of mice infected with P. berghei
Figure 5 showed the effect of the fractions of U. togoensis on serum MDA levels. Oral administration of the ethyl acetate, butanol and residual aqueous fractions of U. togoensis also produced significant (p<0.05) dose dependent decrease in serum MDA levels compared to the control (distilled water treated) group. The standard drug (Chloroquine, 5 mg/kg) significantly (p<0.05) decreased serum MDA levels.
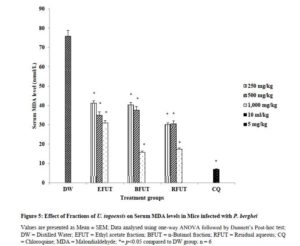
Click to view
Statistical Analysis
Data obtained were in triplicates and expressed as Mean ± SEM (Standard Error of Mean). One-way analysis of variance (ANOVA) was used to determine the differences between groups. Differences between means at 5% level (p < 0.05) were considered statistically significant.
DISCUSSION
Malaria is one of the most prevalent diseases affecting the tropical and subtropical regions of the world with over 3.4 billion people distributed across 92 countries at risk of being infected [1]. The present study sought to examine the effect of administering ethyl acetate, butanol and residual aqueous fractions of the methanol stem bark extract of Uapaca togoensis on antioxidant and lipid peroxidation levels in Plasmodium berghei infected mice.
Preliminary qualitative analysis of the fractions of U. togoensis stem bark showed the presence of phytoconstituents such as steroids, saponins, tannins, alkaloids, terpenoids, and flavonoids. This finding is consistent with other studies [15.18] which reported that the presence of similar phytochemicals in the methanol stem bark extract of U. togoensis. Administration of the fractions of U. togoensis did not produce any physical signs of toxicity, behavioral changes or death in the mice. The oral median lethal dose (LD50) of the fractions was thus estimated to be > 5,000 mg/kg suggesting that the fractions are relatively safe for consumption and for medicinal purposes [25].
The DPPH assay was used to evaluate the hydrogen atom donating ability of the fractions. This was determined by the decolorization of methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) which is purple to yellow color in the presence of antioxidants [26]. Radical scavenging activities are essential to counteract the harmful role of free radicals in several disease states [30]. The results of the study revealed that the butanol fraction showed a better antioxidant activity than the ethyl acetate and residual aqueous fractions; and this suggests that the fractions may possess the ability to transfer electrons or donate hydrogen. Some studies have linked the antiplasmodial/antimalarial activity of medicinal plants including Dodonaea angustifolia [31] and Alchornea laxiflora [32] to their antioxidant effects. Thus, the antioxidant activity exhibited by the fractions of U. togoensis may also contribute to the antimalarial action of the plant.
The invasion of the red blood cells by plasmodium parasite leads to oxidative stress within the parasitized erythrocytes resulting in decreased levels of antioxidant enzymes and an elevation of lipid peroxidation levels [33]. Lipid peroxidation which is a measure of the extent of membrane destruction or changes in structure and function is quantified by the levels of MDA [34]. In the present study, animals that were treated with distilled water (negative control group) had higher levels of MDA in the plasma compared to the fractions pretreated animals and the positive control treated animals. While this confirmed the ability of Plasmodium berghei to cause high levels of lipid peroxidation and thus oxidative stress, the reduced levels of MDA recorded for the fractions reflects the potential of the fractions to inhibit free radical production and reduce lipid peroxidation in plasma. The n-butanol fraction however produced a better reduction in MDA levels than the other fractions.
In the present study, the decrease in SOD, GSH and CAT levels observed in the distilled water treated group could be linked to oxidative inactivation of enzymes. Endogenous antioxidant enzymes (CAT and SOD) and non-enzymatic enzymes (GSH) provide an effective protective system against free radical-induced damage [8]. Treatment of the Plasmodium berhgei infected mice with the fractions significantly increased serum SOD and GSH levels. For CAT, only the butanol fraction produced a dose dependent increase in serum CAT levels compared to the ethyl acetate and residual aqueous fractions. The ethyl acetate fraction was not able to increase serum CAT levels. SOD is a metalloprotein enzyme that catalyzes the dismutation of the superoxide (O2−) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H2O2). Catalase and glutathione shields cells against free radical toxicity by a detoxification process which facilitates the breakdown of hydrogen peroxide to water and molecular oxygen. The results from the present study thus showed that the antioxidant defense capacity of plasma in the animals treated with the fractions was boosted and thus the host cell was protected from oxidative stress and cellular damage.
Many plant phytochemical constituents including saponins, alkaloids, flavonoids and tannins found in the fractions possess good antioxidant activities and are good inhibitors of lipid peroxidation. Thus, the action of these phytoconstituents singly or in combination could be responsible for its antioxidant and antiplasmodial properties observed.
CONCLUSION
Fractions from the stem bark of Uapaca togoensis exhibited varying antioxidant (free radical scavenging) activities when compared to vitamin C in the following order: butanol fraction>residual aqueous fraction>ethyl acetate fraction. The results obtained also suggests that the fractions have the potential to protect P. berghei infected mice from oxidative stress as shown by enhanced CAT, SOD and GSH levels and reduced MDA levels.
ACKNOWLEDGEMENT
The authors are grateful to the laboratory staff of the Department of Pharmacology and Therapeutics Ahmadu Bello University, Zaria for providing the necessary environment and equipment for this study.
CONFLICT OF INTEREST
There is no conflict of interest.
REFERENCES
- World Health Organization, World Malaria Report 2020, World Health Organization, Geneva, Switzerland, 2020. https://www.who.int/publications/i/item/9789240015791
- Ughasoro MD, Okafor HU, Okoli CC. Malaria diagnosis and treatment amongst health workers in University of Nigeria Teaching Hospital Enugu, Nigeria. Niger. J. Clin. Pract 2013; 16:329-33.
- Abdulrazak N, Asiya U, Usman N, Unata I, Farida A, Antiplasmodial activity of ethanolic extract of root and stem back of Cassia sieberiana DC on mice. J. Intercult. Ethnopharmacol 2015; 4(2): 96–101.
- Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol 2004; 34(2): 163–189.
- Ojezele MO, Moke EG, Onyesom I. 2017. Impact of generic antimalarial or Phyllanthus amarus and vitamin co-administration on antioxidant status of experimental mice infested with Plasmodium berghei. Beni-Suef Univ. J. Basic Appl. Sci. 2017; 6: 260-265.
- George BO, Okpoghono, J, Osioma E, Aina OO. Changes in oxidative indices in Plasmodium berghei infected mice treated with aqueous extract of Aframomum sceptrum. Front. Sci. 2012; 2(1): 6-9. doi: 10.5923/j.fs.20120201.02
- Ogbuehi I, Adikwu E, Oputiri D. Effect of Acalypha wilkesiana MuellArg leaf extract on the oxidative indices, liver enzymes and liver integrity of rats infected with Plasmodium berghei. Br. J. Pharmacol. 2014; 5: 68-74.
- Opajobi OA, Ezedom T, Chris-Ozoko LE, Onyesom I. Blood schizonticidal activity of Phyllanthus amarus enhances defense capacity in Plasmodium berghei infected mice. Trop. J. Nat. Prod. 2018; 2:150-157.
- Burkill HM. 1985. The useful plants of west tropical Africa, Volume 3, Royal Botanic Gardens, Kew, UK 1985. p 233 .
- Koné WM, Atindehou KK, Kacou-N’Douba A, Dossod M. Evaluation of 17 medicinal plants from Northern Côte D’Ivoire for their In Vitro activity against Streptococcus Pneumoniae. Afr. J. Tradit. Complement. Altern. Med. 2007; 4:17–22.
- Ajibesin KK. Ethnobotanical survey of plants used for skin diseases and related ailments in Akwa Ibom State, Nigeria. Ethnobot. Res. Appl. 2012; 10: 463-522.
- Kadiri AB, Ayodele AE, Olowokudejo JD, Uchemunefa D. Comparative leaf epidermal morphology of Five West African species of Uapaca bail (Phyllanthaceae Pro Forma Euphorbiaceae). “Niger. J. Bot. 2013; 7: 54-60
- Koné WM, Atindehou KK, Terreaux C, Hostettmann K, Traoré D, Dosso M. Traditional medicine in North Côte-d’Ivoire: Screening of 50 medicinal plants for antibacterial activity. J Ethnopharmacol 2004; 93(1):43-49. doi: 10.1016/j.jep.2004.03.006
- Azokou A, Koné MW, Koudo BG, Tra Bi HF. Larvicidal potential of some plants from West Africa against Culex quinquefasciatus (Say) and Anopheles gambiae Giles (Diptera: Culicidae). J. Vector Borne Dis. 2013; 2: 103–110.
- Omachi AA, Ndukwe GI, Sallau MS, Ayo RG. Phytochemical screening and antimicrobial studies of Uapaca togoensis (pax) stem bark extracts. nt J Eng Sci 2015; 4:24-28.
- Seukep JA, Sandjo LP, Ngadjui BT, Kuete V. Antibacterial activities of the methanol extracts and compounds from Uapaca togoensis against gram-negative multi-drug resistant phenotypes. S. Afr. J. Bot. 2016; 103(1):1-5 doi: 10.1016/j.sajb.2015.08.014
- Kuete V, Sandjo LP, Seukep JA, Zeino M, Mbaveng AT, Ngadjui B, Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards 18 multifactorial drug-resistant cancer cells. Planta Med, 2015; 81(1): 32-8. doi: 10.1055/s-0034-1383362
- Olorukooba AB, Maiha BB, Chindo BA, Ejiofor JI, Hamza AN, Khan F. Preliminary studies on the analgesic and anti-inflammatory activities of the methanol stem bark extract of Uapaca togoensis Pax. (Euphorbiaceae) in rodents. Niger. J. Pharm. Sci. 2015; 14(2): 23-30.
- Olorukooba AB, Maiha BB, Chindo BA, Ejiofor JI, Hamza AN. Antiplasmodial studies on the ethyl acetate fraction of the stem bark extract of Uapaca togoensis (Pax.) Euphorbiaceae in mice. BAJOPAS 2016; 9(1): 191-196.
- Olorukooba AB, Maiha BB, Chindo BA, Ejiofor JI, Hamza AN, Sani MB, Balogun MS. Antimalarial activity of the n-butanol fraction of Uapaca togoensis (Pax) stem bark in mice. Trop. J. Nat. Prod. 2018; 2(1): 29-33.
- Olorukooba AB, Chindo BA, Sani YM. Antioxidant activity of the methanol stem bark extract of Uapaca togoensis (Pax) in mice exposed to Plasmodium berghei NK65. J. Herb Drug; 9(3): 151-159.
- Trease GE, Evans WC. Phytochemistry, In: Textbook of Pharmacognosy 13th Edition, Balliere, Tindall and Cansell Ltd. London; 2002.
- David AF, Philip JR, Simon LC, Reto B, Solomon N. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. 2004; 3:509-520.
- “Principles of laboratory animal care” (NIH Publication no. 25-28, revised 1996).
- Lorke D. A new approach to acute toxicity testing. Arch. Toxicol. 1983; 54:275-287.
- Blios MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 26:1199-1200. http://dx.doi.org/10.1038/1811199
- Aebi HE. Catalase. In: Methods of enzymatic analysis, 3rd edition (Bergmeyer, H. U. Edition) Weinhein, Deerfield Beach, 1983; 273-285 p.
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959; 82: 72-77.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem 1979; 95: 351-8.
- Rahman MM, Islam MB, Biswas M, Khurshid Alam H. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015; 8:621. https://doi.org/10.1186/s13104-015-1618-6
- Amelo W, Nagpal P, Makonnen E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaea angustifolia in Plasmodium berghei infected mice. BMC Compl Alternative Med 2014; 14: 462-9
- Okokon JE, Augustine NB, Mohanakrishnan D. Antimalarial, antiplasmodial and analgesic activities of root extract of Alchornea laxiflora. Pharm. Biol. 2017; 55:1022-1031, doi: 10.1080/13880209.2017.1285947.
- George BO, Okpoghono J, Osioma E, Aina OO. Changes in oxidative indices in Plasmodium berghei infected mice treated with aqueous extract of Aframomum sceptrum. Front. Sci. 2012; 2(1): 6-9. doi: 10.5923/j.fs.20120201.02
- Conrad OA, Dike IP, Agbara U. In vivo antioxidant assessment of two antimalarial plants-Allamamda cathartica and Bixa orellana. Asian Pac J Trop Biomed 2013; 3(5): 388–394. https://doi.org/10.1016/S2221-1691(13)60082-9
,mmmm
