INTRODUCTION
Plants have been successfully used in the treatment of various illnesses over the years and have played been useful in the discovery and development of new drugs. The developing countries exploit herbs and other natural remedies used in the treatment of various ailments as an alternative means to compensate the orthodox that is perceived as unsafe [1]. The plant remedies used in traditional system of treatment around the world are important in the discovery of new antibiotics [2]. Some traditional remedies have successfully been used against antibiotic-resistant strains of bacteria [3]. There is high resistance to the current existing drugs used in the treatment of microbial infection while plants are the cheapest and safer alternative sources of antimicrobials [4-5].
Senna alata (Linn.) belongs to the family Fabaceae. It is an ornamental shrub found in West African forest areas. S. alata is an erect, perennial legume that grows up to about six (6) feet tall. It has dark green even pinnately compound leaves which have orange rachis on stout branches. Each leaf has16-28 leaflets measuring 2-4 inches alternating bilateral in arrangement [6]. Senna alata is indigenous to several regions of the world such as Africa and South America. It is an erect, tropical, annual herb of 0.15 m high with leathery, compound yellowish-green leaves [7]. It thrives in sunny and moist areas and produces a characteristic offensive smell. Ethnopharmacological studies have shown that the leaves of the plant (S. alata) have been used in the treatment of digestion-related ailments such as constipation, abdominal pain and liver diseases [8].
It is locally used in Nigeria in the treatment of several infections, which include ringworm, parasitic skin diseases [9]. The leaves are reported to be useful in treating convulsion, gonorrhoea, heart failure, abdominal pains, oedema and also used as a purgative [10]. A study in Malaysia [11] reported that ethanolic extract of the Senna plant showed high activity against dermophytic fungi.
METHODS
Collection, identification and preparation of Senna alata leaf
The leaves of Senna alata was collected from Kudingi forest, Sabon Gari Local Government Zaria, Kaduna State in the month of August 2016. The sample of Senna alata which included leaves and flower were collected and taken to the Herbarium unit, Department of Botany, Ahmadu Bello University, Zaria for proper identification and authentication and a voucher specimen number 1236 was given. The leaves of the plant collected was dried under shade at room temperature, pulverized to powder using clean motar and pestle, the powder was stored in an air tight container for subsequent use.
Extraction
Extraction of the plant material was done using the method described by Kokate [12]. The pulverized plant material (1 kg) was extracted with 2.5 litres of n-hexane, ethyl acetate and methanol successively in a Soxhlet apparatus.
Phytochemical Screening
The hexane, ethyl acetate and methanol leaf extracts of Senna alata were subjected to qualitative phytochemical screening to identify the phyto-constituents present in the extract, this was carried out according to methods described by Sofowora [13] and Evans [14].
Fractionation of the methanol extract of Senna alata
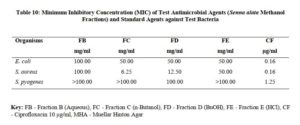
The method described by Woo and co-workers [15] was used to fractionate the methanol extract of Senna alata. The fractionation was done to separate the various components in the crude extracts as extracted from the powdered leaves and five fractions were obtained at the end, fractions “A-E”. A 30 g quantity of the extract was used. The method involves defatting initially with petroleum ether which was labelled as fraction “A” followed by extraction with n-butanol. The butanol and water residue was mixed and shaken vigorously. The two distinct layers were separated and 1% potassium hydroxide (KOH) solution was added to the butanol residue and gently shaken. The aqueous layer was labelled fraction “B” while the butanol portion contains the saponin-rich portion which was labelled as fraction “C”. The KOH solution (alkaline fraction) was neutralise with dilute hydrochloric acid (HCl) and then partitioned with n-butanol. The n-butanol fraction was collected and concentrated and tested for the presences of flavonoids which was the fraction “D”. And the aqueous layer was also concentrated and dried and labelled fraction “E”. The fractions were also tested for amtimicrobial activities. The fractions were having less antimicrobial activity on the organisms that was tested against compared to the crude extract, this could be as a result of synergistic effects of the compounds present in the plant.
Antimicrobial activity
The antimicrobial activity of the leaves extracts of Senna alata was carried out on some fungi and bacteria that causes skin infection. The test organisms were clinical isolates of bacteria: Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes and fungi: Aspergillus fumigatus, Candida albicans, Trichophyton mentagrophytes that were obtained from Microbiology laboratory, Ahmadu Bello University Teaching Hospital, Shika, Zaria.
Preparation of media plates for the antimicrobial sensitivity testing
The culture media used for the analysis were Mueller Hinton Agar (MHA), Mueller Hinton Broth (MHB), Sabouraud Dextrose Agar (SDA) and Sabouraud Dextrose Broth (SDB). The media were used for sensitivity test, determination of minimum inhibitory concentration. (MIC) and minimum bactericidal/ fungicidal concentration (MBC/MFC). All media were prepared according to manufacturer’s instruction and sterilized by autoclaving at 121 °C for 15 minutes.
The standardized inoculum was prepared using normal saline for both bacteria and fungi, the spore suspension was prepared with normal saline and 0.05% Tween 80. The isolates were flooded on sterilized Mueller Hinton agar plates for bacteria and Sabouraud dextrose agar plates for the fungi. Four wells were punch on each inoculated agar plate with a sterile cork borer of 6mm diameter. The wells were properly labelled according to different concentrations of the extract prepared which were 100, 50, 25 and 12.5 mg/ml respectively. Each well was filled up with 100µl of the extract aseptically. Positive controls were set up using ciprofloxacin for the bacteria and fluconazole for the fungi. Negative controls were also set up. The inoculated plates with the extracts were allowed to stay on the bench for one hour this was to enable the extract to diffuse on the agar. The plates were incubated at 37 °C for 24 hours (plates of Mueller Hinton agar) while the plates of Sabouraud dextrose agar were incubated at 30 °C temperature for 4 days. At the end of incubation period, the plates were observed for any evidence of inhibition which appeared as a clear zone that is completely devoid of growth around the wells (zone of inhibition) The diameter of the zones were measured using a transparent ruler calibrated in millimetre and the results was recorded [16-18]
Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration of the extract was determined by using two fold serial dilution method with melted Mueller Hinton agar and melted Sabouraud dextrose agar at 40°C used as a diluent. The agar dilution method involves the incorporation of double strength concentrations of the extract into a double strength of agar medium (molten agar medium), using doubling dilutions starting from (100, 50, 25, 12.5, 6.25, 3.25, 1.56, 0.78, 0.39, 0.19 mg/ml) followed by the inoculation of a standardized microbial inoculum onto the agar plate surface on a sterile filter paper disc. The MIC end point was recorded as the lowest concentration of the extract that completely inhibits growth after 24 hours incubation period at 37°C for bacteria and 4 days incubation period at 30 °C for the fungi [16-18].
Determination of minimum bactericidal/fungicidal concentration (MBC/MFC)
The result from the minimum inhibitory concentration (MIC) was used to determine the minimum bactericidal/fungicidal concentration (MBC/MFC) of the extract. A sterilized wire loop was used to pick up the filter paper disc which showed no sign of growth and was placed in a test tube containing MHB or SDB prepared with 0.3% egg lecithin and 1% Tween 80 with a clear turbidity. The test tubes were covered and incubated at 37 °C for 24 hours and 30 °C for 4 days for the bacteria and fungi respectively. At the end of the incubation period, the tubes were examined/observed for the presence or absence of growth using turbidity as a criterion, the tubes that had no visible sign of growth (turbidity) were considered to be the minimum bactericidal/fungicidal concentration (MBC/MFC) and the result was recorded, this was to determine whether the antimicrobial effects of the extract was bacteristatic/fungistatic or bactericidal/ fungicidal [16].
RESULTS
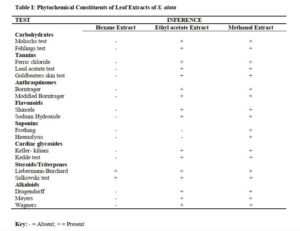
Phytochemical screening of leaf extracts of S. alata revealed the presence of alkaloids, anthraquinones, saponins, tannins, steroids/triterpenes, and flavonoids as shown in Table 1.
Click to view
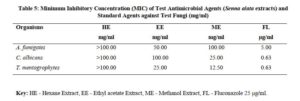
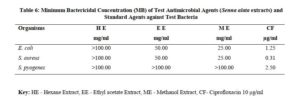
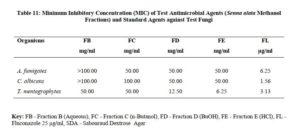
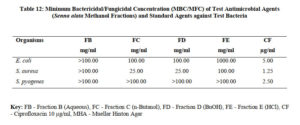
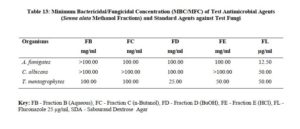
The antimicrobial activities of the hexane, ethyl acetate and methanol extracts of Senna alata were tested on six clinical isolates of Aspergillus fumigatus, Candida albicans, Trichophyton mentagrophytes, Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes. From the results obtained, methanol extracts had the highest activities against most of the organisms among the three extracts. It was also observed that the higher the concentration, the higher the activities of the extract. S. aureus was found to be most sensitive than all the organisms with MIC 6.25mg/ml. Candida albicans showed MIC 25mg/ml, Escherichia coli and Trichophyton mentagrophytes have MIC 12.5mg/ml while A. fumigatus and S. pyogenes were least sensitive with MIC 100mg/ml. The methanol extract was further fractionated and the fractions obtained were also tested for antimicrobial activity on the same organisms. The fractions were having less antimicrobial activity on the organisms that they were tested against compared to the crude extract, and this could be as a result of synergistic effects of the various compounds present in the plant. The results for the zones of inhibition, minimum inhibitory concentrations and the minimum bactericidal/ fungicidal concentrations for the hexane, ethyl acetate and methanol extracts of Senna alata and for the fractions obtained from the Methanol extract of Senna alata are presented in the Tables 2 – 13.

Click to view

Click to view

Click to view
Click to view
Click to view

Click to view

Click to view

Click to view
Click to view
Click to view
Click to view
Click to view
DISCUSSION
Qualitative phytochemical screening revealed the qualitative nature of the extracts, that the present or absent of some classes of phytochemicals. This will provide insight for future experiment using the extract or insight on isolation of the active principle [19].
Phytochemical screening of leaf extracts of S. alata revealed the presence of alkaloids, anthraquinone, saponins, tannins, terpenes, steroids, flavonoids, and carbohydrates this is in line with the work of Owoyale et al [20].
The antimicrobial activity of Senna alata leaf could be attributed to the presence of phytochemicals such as tannins, saponins, alkaloids, steroids and flavonoids. The activity of S. alata against skin ailment was reported [21]
The type of phyto-constituents present in an extract of a plant can be of significant taxonomic keys or marker in the identification of a particular species and distinguishing it from another closely related. Therefore, it is important in delimitation of taxa [22].
The characteristic mixture of these phytochemicals can be produced by each of the plant family, genus and species and can be of taxonomic importance in plant classification [23]. The phytochemical components such as alkaloids, anthraquinones, saponins, tannins, terpenes, steroids and flavonoids, present in the plants have been investigated for antifungal potency [24].
The antimicrobial activities of the hexane, ethyl acetate and methanol extracts of Senna alata was tested on six clinical isolates of Aspergillus fumigatus, Candida albicans, Trichophyton mentagrophytes, Escherichia coli, Staphylococcus aureus and Streptococcus pyogenes using a method described by Alikwe et al [18] with slight modifications. From the results obtained, the methanol extract of S. alata has the highest activity against most of the organisms among the three extracts thus justifies the use of S. alata in the treatment of ringworm and skin diseases in traditional medicine. It was also observed that the higher the concentration, the higher the activity of the extract. Furthermore, S. aureus was found to be most sensitive than all the organisms MIC 6.25mg/ml. A. fumigatus and S. pyogenes were least sensitive MIC 100mg/ml.
The phyto-constituents such as tannins, terpenoids, alkaloids and flavonoids found in methanol extract could be responsible for the antimicrobial activity. These phyto-constituents were previously reported to possess antimicrobial properties in vitro [24-25]. Saponins were also reported as a good antifungal secondary metabolite [26, 24].
The in vitro antimicrobial properties was reported as a result of the presence of phytochemicals such as alkaloids, flavonoids and terpenoids, these phytochemicals and others were reported widely distributed in plants [24-25]. Senna alata possessed a wide range of phytochemicals such as tannins, phenols, saponins and steroids, therefore, the antimicrobial activity displayed by the leaf extracts of the may be attributed to these phytochemicals
CONCLUSION
The phytochemical analysis of the plants indicated the presence of phenolic compounds, tannins, flavonoids, saponins, cardiac glycosides, steroids and triterpenes which may be responsible for the antimicrobial activity of the plants.
The methanol extract of Senna alata leaves possess better antimicrobial activity compared to the hexane and ethyl acetate extracts. This showed that the leaf of S. alata could be useful in the formulation of antiseptic and disinfectant. The plant may be useful in the development of drugs for the treatment of skin infections. The result of this study revealed that the leaf of S. alata has both antibacterial and antifungal properties. This conformed with the reported traditional use of the plant in Northern Nigeria for the treatment or management of bacterial and fungal infections of the skin.
REFERENCES
- Zhu M, Lew KT, and Leung P. Protective effects of plants formula on ethanol induced gastric lesions in rats. Phytother Res, 2002; 16:276-80.
- Okpekon TY, Gleye C, Roblot F, Loiseau P, Bories C, Grellier F. et al. Anti-parasitic activities of medicinal plants used in Ivory Coast. Ethnopharmacol., 2004; 90: 91-97.
- Kone WM, Kamanzi AK, Terreaux C, Hostettmann K, Traore D, and Dosso M. Traditional medicine in North Cote-d’Ivoire screening of 50 medicinal plants for antibacterial activity. Ethnopharmacol., 2004; 93: 43-49.
- Sharif MD, and Banik GR. Status and utilization of medicinal plants in rangamati of Bangladesh. J. Agric. Biol. Sci., 2006; 2(6): 268-273.
- Doughari JH, El-mahmood AM, and Manzara S. Studies on the antibacterial activity of root extracts of Carica papaya Afri. J. Microbiol. Res., 2007; 37- 41.
- Watson L, and Dallwitz MJ. The families of flowering plants. Descriptions and illustrations, identification and information retrieval. 1992; Version: 14th February 2008, http://www.ars-grin.gov2/cgi bin/npg/htm/gnlist.
- Yakubu MT, Adeshina AO, Oladiji AT, Akanji MA, Oloyede OB, Jimoh GA. et al. Abortifacient potential of aqueous extract of Senna alata leaves in rats. Journal of Reprod , 2010; 21(3):163-177.
- Hennebelle T, Weniger B, Joseph H, Sahpaz S, and Bailleul F. Senna alata. Fitoterapia, 2009; 80: 385–393 4.
- Palanichamy S, and Nagaraj S. Antifungal activity of Cassia alata leaf extract. Journal of ethnopharmacology, 1990; 29 (3): 337340.
- Ogunti EG, and Elujoba AA. Laxative activity of Cassia alata. Fitoterapia, 1993; 64 (5): 437-439.
- Ibrahim D, and Osman H. 1995 Antimicrobial activity of Cassia alata from Malaysia. Journal of ethnopharmacology, 1993; 45 (3): 151-156.
- Kokate CK. Practical pharmacognosy, 3rd Edition, Vallabh Prakashan, New Delhi, India. 2003; pp. 107-127.
- Sofowora A. Medicinal plants and traditional medicine in Africa. Spectrum Books Limited, Ibadan, Nigeria. 3rd 2008; Pp. 200-202.
- Evans WC. Trease and evans pharmacognosy, 16th edition, W. B. Saunders Ltd., London, 2009; Pp. 10 – 11.
- Woo SW, shin HH, And Kang KS. Chemistry and pharmacology of Flavon-C-Glycosides from Zizyphus seeds. The Korean J. Pharmacog., 1980; 11(3 and 4): 141-1.
- European Committee for Antimicrobial Susceptibility Testing. EUCAST, Definitive document. terminology relating to the methods for the determination of susceptibility of bacterial to antimicrobial agents. Clinical Microbiology Infection, 2000;(9):503-509.
- Azoro C. Antibacterial activity of crude extracts of Azadirachta indica on Salmonella typhi. World Journal of Biotechnology, 2002;3:347-357.
- Alikwe PC, Ohimain EI, Zige DV, and Angaye TN. Antibacterial activity of ethanol extract of the defatted seed and seed coat of Moringa oleifera. IOSR Journal of Pharmacy and Biological Sciences, 2013; 8(1) 38-41.
- Mishra SB, Mukerjee A, and Vijayakumar M. Pharmacognostic and phytochemical evaluation of leaves extract of Jatropha curcas Journal of Pharmacognosy, 2010; 2:9-14.
- Owoyale JA, Olatunji GA, and Oguntoye SO. Antifungal and antibacterial activities of an ethanolic extract of Senna alata Journal of applied sciences and. environmental Management, 2005; 9: 105-107.
- Makinde AA, Igoli JO, Amal LT, Shaibu SJ, and Garbal A. Antimicrobial activity of Cassia alata. African Journal of Biotechnology, 2007; 6:1509-1510.
- Jonathan G, and Tom JM. Secondary metabolites and the higher classification of angiosperms. Dept of Botany, Univ. of Texas, Austin, TX 78712, USA. Nordic Journal of Botany, 2008; (Impact Factor: 0.6). 03/2008; 3(1):5 – 34. DOI: 10.1111/j.1756-1051.1983.tb01442.x
- Biology Encyclopedia. Secondary metabolites in plants – Biology Encyclopedia, Inc., 2015; pp. 379.
- Sule WF, Okonko IO, Joseph TA, Ojezele MO, Nwanze JC, Alli JA. et al. In-vitro antifungal activity of Senna alata Crude leaf extract. Advances in Applied Science Research, 2010; 1 (2): 14-26.
- Anosike CA, Ogili OB, Nwankwo ON, and Eze EA. Phytochemical screening and antimicrobial activity of the petroleum ether, methanol and ethanol extracts of Ceiba pentandra stem bark. Journal of Medicinal Plants Research, 2012; 6 (46), pp. 5743- 5747.
- Onwuliri FC, and Wonang DL. Studies on the combined antibacterial action of ginger (Zingiber officinale) and garlic (Allium sativum L.) on some bacteria. Nigerian Journal of Botany, 2005; 18: 224-228.
