1. INTRODUCTION
The increasing use of medicinal plants in the treatment of many ailments necessitates the toxicological screening to ascertain their safety among users [1]. Toxicity is a term that describes the level of an adverse effect caused by the interaction of a toxicant and cell(s) of a living organism [2]. Medicinal plants are essential part of healthcare delivery system in resource-poor areas of the world. About 80% percent of the world’s population uses herbal medicine in their healthcare delivery system [3]. The greater demands for resources and time in evaluating the safety and standardizing the use of medicinal plants have led to much emphasis of screening them for efficacy based on traditional claims rather than safety. Toxicological evaluation of medicinal plants is as important as pharmacological validation of ethnomedicinal claims [4]. Other reason that may have led to the shift of attention more on pharmacological validation may stem from the general belief of safety and acceptability of medicinal plants.
Tapinanthus dodoneifolius is a bushy parasitic plant that grows on a wide range of trees around the savannah zone. The plant belongs to the family Loranthaceae. T. dodoneifolius is an important plant use in the treatment of many diseases such as stomach ache, diarrhoea, dysentery, diabetes, epilepsy, hepatitis, hypertension, wounds, cancer etc among the Hausa and Fulani tribes of northern Nigeria. The local names of the plants among different tribes in Nigeria are Etu-lonchi (Nupe), Elozie (Ibo), Kauchi (Hausa) and Afomu Igba (Yoruba) [5]. Other ethnomedicinal uses of Tapinanthus dodoneifolius are treatment of inflammation, fever, infection, dizziness, energy loss, irritability, vertigo and headache. The has a broad spectrum of activity against multidrug resistant bacteria and fungal isolates of farm animals [6, 5]. T. dodoneifolius is also reported to have larvicidal and molluscidal effects. Related Africa mistotle Agelanthus dodonefolins has been reported to have antiplasmodial activity [7]. Despite the dependence on medicinal plants most especially in resource poor areas, there is still sketchy information about their safety [8]. Tapinanthus dodoneifolius is one of such plant. This study was undertaken to evaluate sub-acute toxicity profile of methanol whole extract of Tapinanthus dodoneifolius in Wistar rats.
2. MATERIALS AND METHODS
Collection of Plant
The whole plant of Tapinanthus dodoneifolius was collected from the bush in Sabon Gari Local Government Area of Kaduna State, Nigeria in the month of June, 2018. It was identified and authenticated by Mr. Namadi Sanusi of the Department of Botany, Ahmadu Bello University, Zaria. A voucher number; 0370 was obtained by comparing with existing specimen.
Preparation of Plant Extract
The whole plant of Tapinanthus dodoneifolius was washed, air-dried under shade after which it was grounded to coarse powder using pestle and mortar. About 900 g of the powdered plant material was extracted with 7 L of 70 %v/v methanol for three days using cold maceration with intermittent shaking. The mixture was then filtered using Whatman filter No 1. The filtrate was concentrated and subjected to air drying in crucible. Percentage yield of the methanol extract was calculated and the obtained extract was stored in a desiccator and subsequently referred to as Methanol Extract of Tapinanthus dodoneifolius (METD). Fresh concentrations of the extract were prepared on each day of the experiment by reconstituting in distilled water.
Experimental Animals
Wistar rats of both sexes were obtained from the Animal House Facility of the Department of Pharmacology and Therapeutics Ahmadu Bello University, Zaria. Animals were housed in cages with rodent diet and water ad libitum. Ethical approval with an approval number: (ABUCAUC/2019/006) was obtained from the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC).
Phytochemical Screening
Preliminary phytochemical screening of the methanol whole extract of Tapinanthus dodoneifolius was carried out according to methods described by Trease and Evans and Ayoola [9, 10]
Acute Toxicity Study
The acute toxicity study was carried out according to the Organization for Economic Co-operation and Development (OECD) guidelines 423[11] and fixed dose studies was adopted with 5000 mg/kg body weight as the limit dose. Three rats were fasted for 3-4 hours, and each rat was administered 5000 mg/kg of methanol whole plant extract of Tapinanthus dodoneifolius. Food was withheld for 1 hour post drug administration. Each rat was observed individually for the first 30 minutes and periodically during the first 4 hours and then daily for 14 days for any sign of toxicity, such as tremor, convulsion, salivation, lacrimation, diarrhea, lethargy, sleep, respiratory, behavior pattern, onset and recovery from toxicity and death.
Sub-acute Oral Toxicity Study
The method described by the Organization for Economic Co-operation and Development (OECD) test guideline 407 was adopted for the study [12]. Twenty-four rats (12 males and 12 females) were weighed and grouped into 4 of 3 male and 3 female rats in each group (the males were separated from the females). Group 1, 2, 3 and 4 received 10 mL/kg distil water, 250, 500 and 1000 mg/kg per oral of the extract respectively for 28 consecutive days. Animals were sacrificed under chloroform anaesthesia after being deprived of pellet but had free access to water for 24 hours. Blood samples were collected through cardiac puncture into EDTA and non-heparinized containers for haematological and biochemical analysis respectively. The liver, heart, spleen, stomach, kidney and lungs were collected, weighed and stored in 20 mL of 10 % formyl saline sample bottles (Axiom, Zhanjiang Gong Jong medical technology Co. Ltd, China) and thereafter processed for histopathological studies.
Body Weight
The body weights of the animals were taking at weekly interval during the course of the 28-days treatment. The weight changes were calculated in respect to the initial body weights on day zero.
Organ Weight Index
The heart, lungs, stomach, liver, spleen and kidney were collected, freed of connective tissues and rinsed in normal saline, blotted with filter paper and weighed. The relative organ body weight (ROW) was calculated as: ROW= (organ weight/total body weight) × 100.
Haematological Analysis
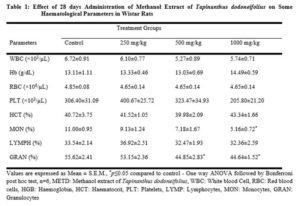
Blood samples were collected and assayed. The parameters assayed include haematocrit (HCT), Hemoglobin (HB), red blood cell (RBC) count, platelet count (PLT), white blood cell (WBC) count, monocytes (MON), lymphocytes (LYMPH), granulocytes (GRAN) using automated haematology analyzer (Mythic 18 by Orphee, Switzerland).
Biochemical Analysis
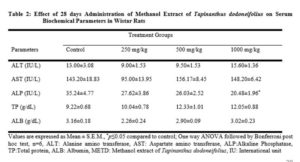
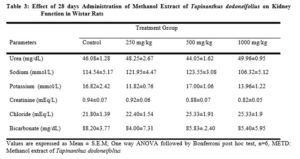
Serum blood samples were analyzed for alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), albumin (Alb), total protein (TP), urea (Bu), creatinine (Crea) using ( Gesan Chem 200, USA) automated machine.
Histopathology
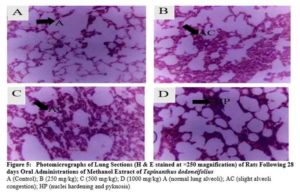
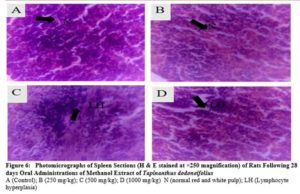
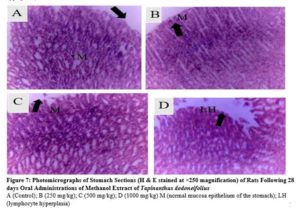
The kidney, liver, heart, lungs, stomach, and spleen were placed into 10 % formalin fixative for histological examinations. Tissue slides were viewed at a magnification of x250 and photomicrographs of the tissues were obtained with the aid of a consultant histopathologist
Statistical Analysis:
Data were expressed as mean ± SEM. One-way and spilt plot analysis of variance (ANOVA) were performed appropriately to compare the differences between means followed by Bonferoni post hoc tests using SPSS version 20.0. A mean difference was considered significant when p ≤ 0.05.
3. RESULTS
The percentage yield of the powdered plant was 14.4%.
Phytochemical Constituents
The preliminary phytochemical screening of METD revealed the presence of alkaloids, cardiac glycosides, saponins, tannins, flavonoids, steroids/triterpenes, terpenoids and carbohydrates. However, anthraquinones was absent.
Acute Toxicity Study
The methanol whole plant extract produced no adverse effect in rats at the dose of 5000 mg/kg. There were no changes in behavior, nature of stool and saliva. There was no mortality observed in the different groups of rats after 7 days. The oral median lethal dose (LD50) of the plant was estimated to be ≥ 5000 mg/kg.
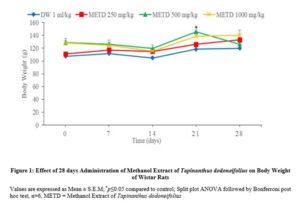
Figure 1: Effect of 28 days Administration of Methanol Extract of Tapinanthus dodoneifolius on Body Weight of Wistar Rats
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
Click to view
4. DISCUSSION
The use of medicinal plants like Tapinanthus dodoneifolius in the treatment of human diseases has enjoyed global acceptability [13]. Comprehensive knowledge about the safety of the plant is yet to be examined despite numerous pharmacological beneficial activities. Therefore, the present study was carried out to ascertain the acute and sub-acute toxicity of Tapinanthus dodoneifolius in experimental animals. The methanol whole plant extract of Tapinanthus dodoneifolius was found to be non-toxic since there was no sign of toxicity and mortality at 5000 mg/kg. The phytochemical constituents such as alkaloids, cardiac glycosides, saponins, tannins, flavonoids, steroids/triterpenes, terpenoids and carbohydrates present in the extract may be responsible for the observed pharmacological activity [14]. A non-significant increase in body weight (Figure 1).was observed which may be due to the nutritional benefit of the plant extract [15]
Haematological parameters are used to ascertain the level of safety of compounds or extract when exposed to human [16, 17] and has been used to explain haematologically-related functions [18]. Changes in haematological parameters are necessary to risk examination due to their predictive values of toxicity [19]. The non-significant changes in RBC, HCT, Hb, and PLT level by METD (Table 1) show that the plant does not affect erythropoiesis and osmotic fragility of the red blood cells [20]. The decrease in the level of monocytes and granulocytes could suggest that the extract may exert toxic effect on the immune system and cause agranolocytosis at dose of 1000 mg/kg and above. The white blood cells (WBC) are the first line of defence which responds to infectious agents, inflammatory process or tissue injury.
The methanol whole plant extract of Tapinanthus dodoneifolius did not show significant changes in AST and ALT enzymes (Table 2), ALP was decreased significantly at dose of 1000 mg/kg suggesting inhibition of the enzyme by the extract.
The serum biochemical assay is used to assess renal and hepatic functions in order to find possible pathological changes due to the extract. Renal and hepatic function analysis is highly useful in the toxicity screening of compounds including medicinal plants as both are important for the excretion and metabolism of drugs by an organism [21]. The liver contains host of enzymes (ALT, AST, ALP) that serve as biochemical markers of liver damages. Once there is a liver damage, these enzymes leak into the serum and show increased activities [22].
The extract at all doses tested could not significantly alter the level of total protein (Table 2). This implies that it is non-toxic to the kidneys and liver. Serum protein and albumin are sensitive indicators of liver function. They are synthesized and metabolized in the liver and serve as a means of assessing hepatocellular injury [23]. Total protein measurement reflects nutritional status and may be used to screen and help diagnose kidney, liver disease and many other conditions. Low total protein levels suggest a liver, kidney disorder or any other condition in which protein is not digested or absorbed properly. High total protein level may be seen with chronic inflammation or liver infections [24].
Measurements of urea, creatinine and uric acid are used to assess renal dysfunction and their normal values indicate absence of renal damage [25]. From the result obtained (Table 3), there was no significant difference among the extract treated groups compared to the control. This suggests that the extract does not seem to possess toxic effect on the kidney. Decrease in organ weight is an expression of toxicity resulting from exposure to toxic substances [26]. The liver; kidney, lungs, stomach, spleen and heart were not adversely affected compared to the control group, throughout the 28 day treatment period (Table 4). Hence, the whole plant extract is nontoxic to the organs in the sub-acute study.
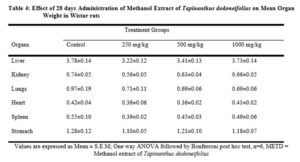
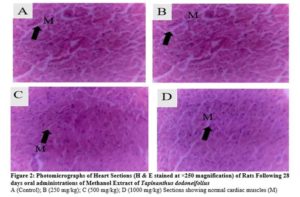
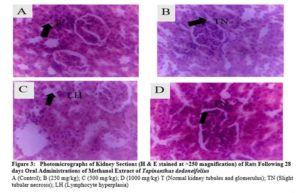
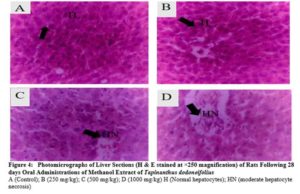
There were no any adverse abnormalities in their gross examinations of the heart, kidney, stomach, spleen, lungs and liver isolated in various group. The histological studies on these organs by the extract did not reveal any pathological changes after treatment compared to the control when administered for 28 days as seen in plate figures 2, 3, 4, 5, 6 and 7.
5. CONCLUSION
The methanol whole plant extract of T. dodoneifolius does not seem to possess the toxic effects that could affect its ethnomedicinal use.
ACKNOWLEDGEMENT
The authors are grateful to the entire staff of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria, Nigeria for providing the enabling environment to carry out this work.
CONFLICT OF INTERESTS
There are no conflicts of interest.
REFERENCES
- Asgarpanah J, Ramezanloo F. Chemistry, pharmacology and medicinal properties of Peganum harmala African Journal of. Pharmaceutical Pharmacology 2012; 6(22):1573-1580
- Syahmi ARM, Vijayarathna S, Sasidharan S, Latha, LY, Kwan YP, Lau YL, Shin LN, Chen Y. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis (Oil palm leaf) methanol extract. Molecules2010; 15:8111– 8121.
- Peter JH. The Role of Plants in Traditional Medicine and Current Therapy. The Journal of Alternative and Complementary Medicine. 1995; 1(2): 131-143.
- Sofowora A. Medicinal plants and Traditional Medicine in Africa.2nd edition. Spectrum Books Ltd, Ibadan, Nigeria; 1993. 289 p.
- Deeni YY, Sadiq NM. Anti-microbial Properties and Phytochemical constituents of Tapinanthus dodoneifolius (DC) Danser (Loranthaceae): An Ethno medicinal Plant of Hausa land, Northern Nigeria. Journal of Ethnopharmacology 2002; 83(3):235-40.
- Cepleanu F, Hamburger MO, Sordat B. Screening of tropical medicinal plants for molluscidal, larvicidal, fungicidal and cytotoxic activities and brine shrimp toxicity. International Journal of Pharmacognosy 1994; 32:294-307.
- Builders MI, Oguru MO, Aguiyi C. Anti-plasmodial potential of the African mistletoe: Agelanthus dodoneifolius Polh and Wiens. Indian Journal on Pharmaceutical Sciences 2012; 74(3):223-229.
- World Health Organization (WHO). Traditional Medicine Fact sheet 2008; No. 134. Retrieved from: who.int/mediacentre/factsheets/fs134/en/
- Evans, W.C. (2002). Trease and Evans Pharmacognosy. 15th Edition, Elsevier, India. p. 27, 46, 183-184, 289-291, 411-413, 434, 485-486.
- Ayoola GA, Coker HAB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, Atangbayila TO. Phytochemical Screening and Antioxidant Activities of some Selected Medicinal Plants used for Malarial Therapy in South Western Nigeria. Tropical Journal of Pharmaceutical Research 2008; 7:1019-1024.
- Organisation for Economic Cooperation and Development (OECD). Guidelines for the Testing of Chemicals/Section 4: Health Effects Test No. 423. Acute oral toxicity-acute toxic class method, Paris. 2002.
- Organisation for Economic Cooperation and Development (OECD). OECD guidelines for testing of chemicals: Guideline 407: Repeated dose 28-day oral toxicity in rodents. Office of Economic and Community Development, Paris. 2008.
- Afolabi SO, Akindele AJ, Awodele O, Anunobi CC, Adeyemi OO. A 90 day chronic toxicity study of Nigerian herbal preparation DAS-77 in rats. Complementary and Alternative Medicine 2012; 12:79.
- Edeoga HO, Okwu E, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology2005; 4(7):685-688.
- Ezeonwumelu JOC, Julius AK, Muhoho CN, Ajayi AM, Oyewale AA, Tanayen JK, Balogun SO, Ibrahim A, Adzu B, Adiukwu CP, et al. Biochemical and Histological Studies of Aqueous Extract of Bidens pilosa leaves from Ugandan Rift Valley in Rats.British Journal of Pharmacology and Toxicology2011; 2(6):302-309.
- Agbaje EO, Adeneye AA, Daramola AO. Biochemical and Toxicological Studies of Aqueous Extract of Syzigium Aromaticum (L.) & Perry (Myrtaceae) in Rodents. African Journal of Traditional, Complementary Alternative Medicine2009; 6(3): 241–254.
- Ibrahim MB, Sowemimo AA, Sofidiya MO, Badmos KB, Fageyinbo MS, Abdulkareem FB, Odukoya OA.Sub-acute and chronic toxicity profiles of Markhamia tomentosa ethanolic leaf extract in rats. Journal of Ethnopharmacology 2016; 193:68-75.
- Yakubu MT, Akanji MA and Oladiji AT. Haematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis Pharmacognosy Magazine 2007; 3: 34– 38.
- Olso H, Betton G, Robinson D,Thomas K, Monro A, Kolaja G, Lilly P, Sanders J Sipes G, Bracken W, Dorato M, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regulation of Toxicology and Pharmacology2000; 32, 56–67.
- Guyton AC, Hall JE. TextbookofMedicalPhysiology, 11thed.Elsevier Saunders, USA. 2006.1152 p
- Olorunnisola OS, Bradley G and Afolayan AJ. Acute and subchronic toxicity studies of methanolic extract of Tulbaghia violacea rhizomes in Wistar rats.African Journal of Biotechnology2012; 11: 14934–14940.
- Akanji MA, Nafiu MO, Yakubu MT. Enzyme activities and histopathology of selected tissues in rats treated with potassium bromate. African Journal on Biomedical Research 2008; 11: 87-95.
- Ganong WF. Review of Medical Physiology. 20th edition. New York: Lange Medical Books, McGraw Hill Companies International. 2016. p. 500–515
- Ighodaro I, Silvanus IS, Vincent O, Lucy A. Chronic Toxicity Studies of Aqueous Leaf Extract of Voacanga africana in Wistar Rats. Journal of Appl. Sci. Environ. Management 2015; 19 (4) 639-646.
- Davis ME and Bredt ND. Renal methods for toxicity, in Principles and Methods of Toxicology, Raven Press. New York, USA. 1994
- Adeyemi OO, Akindele AJ, Nwumeh KI. Acuteandsubchronictox- icological assessmentof Byrsocarpus coccineus And Thonn. (Con-naraceae) aqueous leaf extract. International Journal of Applied Research Natural Product 2010; 3, 1–11.
